Abstract
Polycythemia vera (PV) is a chronic myeloproliferative neoplasm associated with JAK2 mutations (V617F or exon 12) in almost all cases. The World Health Organization has defined the criteria for diagnosis, but it is still unclear which parameter (hemoglobin or hematocrit) is the most reliable for demonstrating increased red cell volume and for monitoring response to therapy; also, the role of bone marrow biopsy is being revisited. PV is associated with reduced survival because of cardiovascular complications and progression to post-PV myelofibrosis or leukemia. Criteria for risk-adapted treatment rely on the likelihood of thrombosis. Controlled trials have demonstrated that incidence of cardiovascular events is reduced by sustained control of hematocrit with phlebotomies (low-risk patients) and/or cytotoxic agents (high-risk patients) and antiplatelet therapy with aspirin. Hydroxyurea and interferon may be used as first-line treatments, whereas busulfan is reserved for patients that are refractory or resistant to first-line agents. However, there is no evidence that therapy improves survival, and the significance of reduction of JAK2 mutated allele burden produced by interferon is unknown. PV is also associated with a plethora of symptoms that are poorly controlled by conventional therapy. This article summarizes my approach to the management of PV in daily clinical practice.
Introduction
I will take advantage of the history of two gentlemen that I encountered in the office some years apart to illustrate how I manage subjects referred for the investigation of erythrocytosis and are then found to have polycythemia vera (PV) rather than relative or secondary erythrocytosis (Table 1).1 Therefore, this manuscript is not intended to provide a thorough description of the disease or to address still debated topics,2-8 but rather to focus on daily practical aspects.
Relative and secondary erythrocytosis commonly encountered in the clinical practice
| Mechanism . | Found in . |
|---|---|
| Reduction of plasma volume (relative erythrocytosis) | Acute (protracted vomiting or diarrhea, severe burns, protracted fever, diabetic ketoacidosis) and chronic (prolonged and inappropriate use of diuretics, Gaisböck syndrome) conditions |
| Appropriately increased sEPO levels | Chronic obstructive pulmonary disease, cyanotic heart disease, smokers, people living at high altitudes, sleep apnea, obesity, eventually associated with sleep apnea, and drugs (androgens and corticosteroids); doping with recombinant preparation of human EPO |
| Inappropriately increased sEPO levels | Renal cell carcinoma, non-neoplastic renal lesions (cysts, hydronephrosis, and severe stenosis of the renal artery), hepatocellular carcinoma, uterine fibroma, meningioma, cerebellar hemangioblastoma, other tumors (Wilms’ tumor, ovarian, carcinoid, and pituitary adenoma), and following renal transplantation |
| Mechanism . | Found in . |
|---|---|
| Reduction of plasma volume (relative erythrocytosis) | Acute (protracted vomiting or diarrhea, severe burns, protracted fever, diabetic ketoacidosis) and chronic (prolonged and inappropriate use of diuretics, Gaisböck syndrome) conditions |
| Appropriately increased sEPO levels | Chronic obstructive pulmonary disease, cyanotic heart disease, smokers, people living at high altitudes, sleep apnea, obesity, eventually associated with sleep apnea, and drugs (androgens and corticosteroids); doping with recombinant preparation of human EPO |
| Inappropriately increased sEPO levels | Renal cell carcinoma, non-neoplastic renal lesions (cysts, hydronephrosis, and severe stenosis of the renal artery), hepatocellular carcinoma, uterine fibroma, meningioma, cerebellar hemangioblastoma, other tumors (Wilms’ tumor, ovarian, carcinoid, and pituitary adenoma), and following renal transplantation |
Gaisböck syndrome is an ill-defined benign syndrome seen in obese, hypertensive, and middle-age male smokers presenting with modest increase of Hct not corresponding to increased red cell mass (RCM).
Case 1 was a 47-year-old dentist who presented in early 2012 with a routine blood test showing the following: hemoglobin (Hb), 233 g/L; hematocrit (Hct), 68.9%; mean corpuscular volume, 81 fL; leukocytes, 4.4 × 109/L; platelets, 145 × 109/L; hyperuricemia, and subnormal ferritin levels. He said he felt “perfectly well,” “I am working 10 hours a day,” but during the interview, he acknowledged to have occasional scotoma and tinnitus in the last weeks; a physical examination was unremarkable except for mild hypertension. I ordered additional blood tests and prescribed aspirin; phlebotomies were initiated the same day.
Case 2 was a 68-year-old retired man, who was a heavy smoker, seen in 2003, in which we discovered the following abnormalities: Hb, 178 g/L; Hct, 57.7%; mean corpuscular volume, 74 fL; leukocytes, 13.6 × 109/L; platelets, 535 × 109/L. One year before, he had an acute myocardial infarction; an Hb and Hct of 165 g/L and 52.6%, respectively, were not further investigated. He was on antihypertensive medications and antidiabetics and was taking aspirin. He complained of tiredness, itching, and paresthesia. The spleen was palpable at 5 cm from the left costal margin (LCM). A diagnostic path was initiated, and phlebotomies were prescribed.
At presentation
The extent of clinical and laboratory information collected at baseline in a subject with unexplained erythrocytosis may influence the diagnostic approach and, once PV is eventually diagnosed, the assessment of disease-associated risk and subsequent therapeutic decisions. Sixty percent of PV patients are >60 years of age, and 10% are <40 years of age; there is no gender preference.9 Erythrocytosis may be discovered serendipitously (case 1) or during investigation of vague upsets, such as unexplained weakness, pruritus (typically after a shower with warm water, but eventually during physical exercise, at bed time, or when changing cloths), headache, paresthesia, tinnitus, blurred vision, arthralgia, abdominal discomfort, and excess sweating.10 In 10% to 15% of cases, the diagnosis of PV occurs concurrently with, or shortly after, a thrombotic event (case 2); however, such a presentation is less common than in the past because of the widespread use of laboratory screening tests and greater awareness by practitioners.11 At the first visit, I invite the subject to freely describe her/his complaints, and then I ask a basic list of questions that provide information on personal and familial medical history, lifestyle, concurrent medications, and symptoms typically associated with PV (Table 2). It is important to interrogate the patient minutely because many causes of secondary erythrocytosis can be elucidated by history, and conversely, some PV-associated manifestations may have gone underestimated. The myeloproliferative neoplasm (MPN) self-assessment12 questionnaire has been devised to measure the burden of disease-associated symptoms,13 but outside clinical trials, its use is still limited. Physical examination may discover conjunctival plethora and ruddy cyanosis; a palpable spleen is found in ∼40% of cases, whereas massive splenomegaly and hepatomegaly are infrequent. Most subjects are already on antihypertensive drugs or have mild hypertension.
List of basic questions directed to a subject first investigated for erythrocytosis and during follow-up if PV is eventually diagnosed
| In relation to . | Factors . |
|---|---|
| Personal/medical history | Work exposure, past (resolved) diseases, including neoplasia, surgical interventions, past cardiovascular events and hemorrhage, if menopausal (woman), and altitude (where you live) |
| Family | Relatives with a diagnosis of MPN or suggestive of MPN, with other hematologic neoplasia or disorders; relatives with unexplained erythrocytosis (even vague); and relatives with thrombotic events in unusual sites and/or at an unusual age |
| Lifestyle | Smoking, physical activity, dietary habits, in general, did you modify your lifestyle as we discussed previously?; and nocturnal apnea (ask the spouse) |
| Concomitant comorbidities | Other diseases, in general; and in particular, hypertension, diabetes, hypercholesterolemia, hypertriglyceridemia, hyperuricemia, and gout |
| Medications | Use of antihypertensive agents, especially diuretics; use of androgens; chronic use of corticosteroids; use of antiplatelet aggregants or anticoagulants and why; and compliance of prescribed medications, any |
| Contraception | Past and current use of contraceptives |
| Pregnancies | Previous pregnancies and outcomes; abortions and/or miscarriages; and planning for future pregnancies |
| Actual symptoms | “Constitutional” symptoms (drenching night sweats, weight loss, unexplained fever >37.5°C); pruritus and characteristics (when, how long, how do you manage it?); vasomotor symptoms (headache, dizziness, tinnitus, acral paresthesias, erythromelalgia, skin reddening, and concentration problems); muscle and joint aches; bone pain; abdominal discomfort, early satiety; and level of tiredness/fatigue and how it affects daily activities |
| In relation to . | Factors . |
|---|---|
| Personal/medical history | Work exposure, past (resolved) diseases, including neoplasia, surgical interventions, past cardiovascular events and hemorrhage, if menopausal (woman), and altitude (where you live) |
| Family | Relatives with a diagnosis of MPN or suggestive of MPN, with other hematologic neoplasia or disorders; relatives with unexplained erythrocytosis (even vague); and relatives with thrombotic events in unusual sites and/or at an unusual age |
| Lifestyle | Smoking, physical activity, dietary habits, in general, did you modify your lifestyle as we discussed previously?; and nocturnal apnea (ask the spouse) |
| Concomitant comorbidities | Other diseases, in general; and in particular, hypertension, diabetes, hypercholesterolemia, hypertriglyceridemia, hyperuricemia, and gout |
| Medications | Use of antihypertensive agents, especially diuretics; use of androgens; chronic use of corticosteroids; use of antiplatelet aggregants or anticoagulants and why; and compliance of prescribed medications, any |
| Contraception | Past and current use of contraceptives |
| Pregnancies | Previous pregnancies and outcomes; abortions and/or miscarriages; and planning for future pregnancies |
| Actual symptoms | “Constitutional” symptoms (drenching night sweats, weight loss, unexplained fever >37.5°C); pruritus and characteristics (when, how long, how do you manage it?); vasomotor symptoms (headache, dizziness, tinnitus, acral paresthesias, erythromelalgia, skin reddening, and concentration problems); muscle and joint aches; bone pain; abdominal discomfort, early satiety; and level of tiredness/fatigue and how it affects daily activities |
Diagnostic workup
The diagnostic workup of erythrocytosis is now greatly facilitated by the almost perfect association of PV with a JAK2 mutation.14 The JAK2V617F mutation accounts for 95% of World Health Organization (WHO)-defined PV15 ; a further 2% to 4% of patients harbor mutations in JAK2 exon 12.16,17 Rare LNK18 mutations occur in patients with isolated erythrocytosis, but whether these are true PV is unclear.19,20 Because the JAK2V617F mutation is found also in ∼60% of essential thrombocythemia (ET) and primary myelofibrosis (PMF), its presence unequivocally identifies a MPN and excludes secondary erythrocytosis but does not help define the phenotype.21 Conversely, exon 12 mutations are reported exclusively in PV. Several qualitative or quantitative assays for the JAK2V617F mutation have been developed.21-23 The median mutated allele burden in PV is ∼50%, greater than in most ET patients (∼20%) and comparable to PMF, but individual values range from 1% to 100%. Although measurement of allele burden is not informative at diagnosis, I use a quantitative assay because of greater sensitivity and reproducibility. I do not measure the JAK2V617F allele burden during the follow-up or for assessing response to cytotoxic therapy24 because the results will not influence therapeutic decisions. However, in patients that are suspected to evolve to post-PV myelofibrosis (PPV-MF), I repeat the test to document accumulation of mutated alleles that usually accompanies transition to PPV-MF.25-28 At present, there is no evidence that assessing other MPN-associated mutations14 provides prognostic information, unlike in PMF patients.29-31 I routinely order quantification of serum erythropoietin (sEPO) levels before phlebotomy is started to avoid fluctuations.
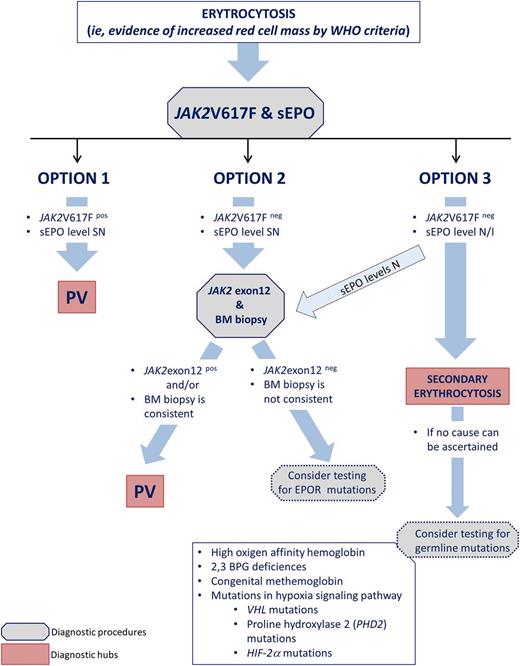
Once results of JAK2 and sEPO tests are available, I am faced with 3 options (Figure 1).
Option 1: The JAK2V617F mutation is detected, and sEPO levels are subnormal: the diagnosis is PV. According to the WHO 2008 criteria,15 bone marrow (BM) biopsy is not required to substantiate the diagnosis, but I discuss with the patient why I suggest to do it: to assess the degree of reticulin fibrosis and perform karyotype analysis. Of note, in the proposed revised diagnostic criteria (Table 332 ), BM biopsy is mandatory because it helps differentiate early PV with concomitant thrombocytosis from ET,33 and in patients with increased Hb levels, yet below the WHO threshold, BM biopsy may be diagnostic.34 A grade 1 reticulin fibrosis is found in 15% of PV patients at baseline and does not imply an alternative diagnosis27 ; however, patients with fibrosis may have a more advanced phase and be more prone to develop PPV-MF.35 An abnormal karyotype has prognostic relevance.9 I do not repeat the biopsy serially unless evolution to PPV-MF or acute leukemia is suspected.
Option 2: The JAK2V617F mutation is absent and sEPO levels are subnormal: then, I order a test for JAK2 exon 12 mutation and BM biopsy. A detectable JAK2 exon 12 mutation will establish diagnosis of PV; otherwise, BM biopsy may support diagnosis of a (rare) JAK2 unmutated PV by WHO 2008 criteria.15 I very rarely use the endogenous erythroid colonies test for diagnostic purposes (Table 3). In case exon 12 mutations are absent and BM biopsy is inconclusive, germ-line mutations of the EPO receptor may be considered, particularly if family history is evocative.
Option 3: JAK2V617F mutation is absent and sEPO levels are normal/increased: secondary erythrocytosis is likely (Table 1). It must, however, be considered that according to a recent study, only 82% of PV patients had subnormal EPO levels, 16% had normal levels, and 2% had increased levels. Therefore, in the case of normal sEPO levels, it is still appropriate to order JAK2 exon 12 genotyping and eventually proceed to BM biopsy. If no cause of secondary erythrocytosis can be demonstrated, a rare congenital erythrocytosis may be suspected (Figure 1).
A simplified flowchart for a diagnostic approach to PV that I use for the investigation of erythrocytosis. Diagnostic procedures indicated with a dotted border require a high level of suspicion and, preferably, reasonable evidence of familial history. These genetics tests are complex, costly, and should be performed in a specialized laboratory. BPG, erythrocyte 2,3-biphosphoglycerate; EPO-R, erythropoietin receptor; HIF-2a, hypoxia-inducible factor 2a; N/I, normal/increased; PDH2, prolyl hydroxylase domain protein 2; SN, subnormal; VHL, von Hippel-Lindau.
A simplified flowchart for a diagnostic approach to PV that I use for the investigation of erythrocytosis. Diagnostic procedures indicated with a dotted border require a high level of suspicion and, preferably, reasonable evidence of familial history. These genetics tests are complex, costly, and should be performed in a specialized laboratory. BPG, erythrocyte 2,3-biphosphoglycerate; EPO-R, erythropoietin receptor; HIF-2a, hypoxia-inducible factor 2a; N/I, normal/increased; PDH2, prolyl hydroxylase domain protein 2; SN, subnormal; VHL, von Hippel-Lindau.
Criteria for the diagnosis of PV
| Current (2008) WHO criteria15 . | Proposal for revised criteria (2014)32 (pending WHO endorsement) . |
|---|---|
| Major criteria | |
| 1. Hb >18.5 g/dL in men, >16.5 g/dL in women, or evidence of increased red cell volume, or* | 1. Hb >16.5 g/dL in men, >16.0 g/dL in women, or Hct >49% in men, >48% in women |
| 2. Presence of JAK2V617F mutation or other functionally similar mutations (e.g., JAK2 exon 12 mutation) | 2. BM biopsy showing trilineage myeloproliferation and pleomorphic megakaryocytes |
| 3. Presence of JAK2 mutation | |
| Minor criteria | |
| 1. BM biopsy showing trilineage myeloproliferation | 1. sEPO level below the normal reference range |
| 2. sEPO level below the normal reference range | |
| 3. Endogenous erythroid colony growth | |
| PV diagnosis requires meeting either both major criteria and 1 minor criterion or the first major criterion and 2 minor criteria | PV diagnosis requires meeting all 3 major criteria or the first 2 major criteria and the minor criterion |
| Current (2008) WHO criteria15 . | Proposal for revised criteria (2014)32 (pending WHO endorsement) . |
|---|---|
| Major criteria | |
| 1. Hb >18.5 g/dL in men, >16.5 g/dL in women, or evidence of increased red cell volume, or* | 1. Hb >16.5 g/dL in men, >16.0 g/dL in women, or Hct >49% in men, >48% in women |
| 2. Presence of JAK2V617F mutation or other functionally similar mutations (e.g., JAK2 exon 12 mutation) | 2. BM biopsy showing trilineage myeloproliferation and pleomorphic megakaryocytes |
| 3. Presence of JAK2 mutation | |
| Minor criteria | |
| 1. BM biopsy showing trilineage myeloproliferation | 1. sEPO level below the normal reference range |
| 2. sEPO level below the normal reference range | |
| 3. Endogenous erythroid colony growth | |
| PV diagnosis requires meeting either both major criteria and 1 minor criterion or the first major criterion and 2 minor criteria | PV diagnosis requires meeting all 3 major criteria or the first 2 major criteria and the minor criterion |
Or Hb or Hct >99th percentile of reference range for age, gender, and altitude of residence or Hb >17 g/dL in men, >5 g/dL in women, if associated with a documented and sustained increase of ≥2 g/dL from a person’s baseline value that cannot be attributed to correction of iron deficiency, or RCM >25% above mean normal predicted level.
Returning to our patients: establishing diagnosis
Case 1
The subject had undetectable sEPO levels and the JAK2V617F mutation was not found; a search of JAK2 exon 12 mutations also was unsuccessful. A BM biopsy showed increased age-adjusted cellularity, markedly expanded erythroid compartment, normal myeloid, and slightly increased megakaryocytic lineage; reticulin fibrosis was absent. The karyotype was normal. A tentative diagnosis of JAK2 unmutated PV was made that was modified to JAK2 exon 12-mutated PV 4 months later when a N542-E543del abnormality was discovered by next-generation sequencing.
Exon 12 mutations can be demonstrated in granulocyte DNA by high-resolution melting analysis (sensitivity, 5-10%)36-38 and standard sequencing (sensitivity, 15-20%),39 but in some cases, the mutant allele burden is low enough to prevent detection.16 In this patient, I stubbornly ordered the search for exon 12 mutations by next-generation sequencing; this approach was successful, but I admit that this is not standard procedure. The BM of JAK2 exon 12-mutated patients may lack features typically associated with JAK2V617F-mutated PV; cellularity may be only moderately increased, and myeloid and megakaryocyte lineages often result normal.40
Case 2
The patient had subnormal sEPO levels and was JAK2V617F mutated with an allele burden of 40%. The BM biopsy showed trilineage proliferation with pleomorphic megakaryocytes and a grade 1 reticulin fibrosis. The karyotype was normal. The blood film showed neutrophil leukocytosis without immature forms. A diagnosis of JAK2V617F mutated PV was made.
Although the best method for demonstrating increased RCM is isotope determination using chromium-51 (Cr-51)–labeled erythrocytes, the test is now performed in a limited number of centers and/or in selected situations because of its intricacy and cost. Whether Hb or Hct, in the context of a JAK2 mutation, is the best surrogate of increased RCM is outside the purpose of this manuscript.2,6,7,41 Recent studies highlighted that in JAK2V617F-mutated subjects with thrombocytosis and an Hb level of ≥165 and ≥160 g/L in men and women, respectively, a diagnosis of early PV rather than ET should be considered. The above-threshold levels are incorporated in the newly proposed revision of WHO criteria (Table 3). In this context, BM biopsy helps to distinguish early forms of PV from ET.33
Communicating the diagnosis
The term “neoplasm,” introduced by the WHO in 2008, causes upset and anxiety; therefore, it is important to reassure the patient that PV is a chronic disorder that, if carefully managed, has projected life expectation “not far” from normal. However, survival is reduced compared with the general population: a recent study reported a median survival of 14.1 years.9 I mention the risk of late progression to PPV-MF (15-20%)42 or leukemia (3-5%)9 and how current treatments are unable to prevent either. I stress the need to adopt lifestyle interventions for reducing the risk of vascular complications, including control of body weight, physical exercise, adherence to antihypertension, antidiabetes, and antihypercholesterolemia/hyperlipidemia medications, avoidance of oral contraceptives, and situations that carry over a risk of bleeding. Major hemorrhages are reported in 2% to 8% of patients.43,44 I am particularly determined in asking the patient to stop smoking, which is associated with an increased risk of arterial thrombosis.43,45 Many patients ask if PV is a genetic disease. I clarify that there is no evidence of inheritance, although familial predisposition is supported by epidemiologic data46 and a predisposition allele (JAK2 46/1),47-49 and overemphasize that there is no reason to check relatives for mutations. I eventually discuss about strategies for future pregnancies and surgery. Finally, I give the patient an informative booklet containing a lay description of the disease, a list of common questions and answer, and links to scientific and advocacy groups.50
Prognostic stratification for risk-adapted therapy
A prognostic model for overall survival, based on age, leukocytosis, and venous thrombosis, has been developed recently; it separates patients into 3 groups with median survival of 28, 19, and 11 years.9 However, risk-adapted therapy in PV is based on an estimate of the likelihood of thrombotic complications and not necessarily on survival and hematologic progression; controlled evidence indicates that appropriate therapy can reduce the rate of thrombosis while there is no suggestion of an effect on disease natural history. In the prospective European Collaboration on Low-Dose Aspirin in PV study of patients diagnosed by the PV Study Group criteria, the cumulative rates of nonfatal thrombosis and cardiovascular mortality were 3.8 and 1.5 events per 100 patients per year,43 respectively. The lower rate of thrombosis (2.7 per 100 patients per year; 17% arterial and 12% venous) reported in patients diagnosed by the WHO 2008 criteria in the controlled Cytoreductive Therapy in PV study51 reflects a more homogenous series of patients and improved disease management. Age >60 years and previous thrombosis are the criteria used to stratify patients into low- and high-risk categories (Table 4). Although generic cardiovascular risk factors deserve corrective interventions, they are not formally considered during risk stratification; the same goes for leukocytosis45,52 and JAK2V617F allele burden.25
Criteria for risk stratification in patients with PV and risk-adapted therapy
| Risk category . | Risk variables . | Therapy . |
|---|---|---|
| Low | Age <60 years old; and no thrombosis history | Phlebotomy and correction of CV risk factors, and aspirin |
| High | Age ≥60 years old and/or thrombosis history | Cytoreduction, and correction of CV risk factors, and aspirin*, plus/minus phlebotomy† |
| Risk category . | Risk variables . | Therapy . |
|---|---|---|
| Low | Age <60 years old; and no thrombosis history | Phlebotomy and correction of CV risk factors, and aspirin |
| High | Age ≥60 years old and/or thrombosis history | Cytoreduction, and correction of CV risk factors, and aspirin*, plus/minus phlebotomy† |
The most frequent thrombosis in PV includes stroke, myocardial infarction, peripheral arterial thrombosis, transient ischemic attack, peripheral vein thrombosis, pulmonary embolism, and thrombosis in unusual venous districts. Aspirin is low-dose, i.e., 81 to 100 mg daily. CV, cardiovascular.
Or, depending on the thrombosis type, oral anticoagulation instead of aspirin.
Phlebotomy may be associated with cytoreductive therapy to, initially, speed up and, later, help to maintain the target level of Hct.
Therapy goals and therapy options
The goal of therapy in PV is primarily to reduce the risk of thrombosis without increasing the bleeding tendency and enhancing the intrinsic potential of hematologic progression and, secondarily, to ameliorate symptoms, particularly vasomotor manifestations.53 The patient has to be made aware that no drug is able to cure the disease and that therapy is personalized to maximize benefits while limiting potential risks. I inform patients of common side effects and alert to report promptly; I also instruct to avoid iron supplementation.
The cornerstone of treatment is the correction of abnormal blood viscosity associated with the increased red cell volume that can be pursued with phlebotomy only in the low-risk and/or with cytotoxic drugs in the high-risk category (Table 4).53,54 The optimal target of Hct that I adopt is <45%, although others recommend <42% for women.7,55 In the Cytoreductive Therapy in PV trial, patients maintained at Hct <45% had a significantly lower rate of cardiovascular death and major thrombosis than those at 45% to 50%.51 In patients with splanchnic vein thrombosis (SVT), most of whom are women, I prefer to maintain a Hct <42%. Phlebotomies are performed twice weekly at the beginning (alternate days if Hct is ≥60%) until the Hct is <45%, and then the frequency is adjusted based on blood counts measured at 4- to 8-week intervals. Young people tolerate well the removal of 350 to 450 mL of blood, whereas in the elderly, it is advisable to withdraw smaller volumes. In high-risk patients, I prescribe hydroxyurea as first-line cytoreduction starting at 500 mg twice a day, and then titrate the dose on the basis of the target Hct and hematologic toxicity. The European LeukemiaNet/International Working Group for Neoplasms Research and Treatment response criteria,56 based on combined hematologic, clinical, and molecular end points, are appropriate in the setting of clinical trials but are poorly applicable in current practice.57 I do not use pipobroman,58 which has been associated with increased leukemic transformation,59 nor chlorambucil or radioactive phosphorus.60 Conversely, no controlled study has firmly implicated hydroxyurea as being leukemogenic in PV, although the combination with alkylating agents may increase the risk.43 Conventional and pegylated interferon-α controls blood counts in most patients61-64 and may be the first-line therapy, particularly in younger patients.53,61,62 However, interferon-α is not approved for PV, and its availability is restricted in some countries. Interferon preferentially targets JAK2V617F-mutated cells,65 causing progressive decrease of mutated allele burden and eventually its complete disappearance63,66 ; however, founder clones harboring TET2 mutations are not targeted by interferon.67 The usual maintenance dose is 3 × 106/MU 3 times a week (conventional) or 90 to 180 µg weekly (pegylated), but it is titrated individually based on efficacy and toxicity. Side effects (autoimmune disorders, flu-like manifestations, depression, heart, and ocular disease) lead to permanent discontinuation in 20% to 40% on conventional and 20% to 25% on pegylated interferon. However, both hydroxyurea and interferon are poorly effective in relieving symptoms, although the latter may help some patients with severe itching68 ; also, the effects on splenomegaly are usually modest and transient. I do not consider thrombocytosis (unless extreme, >1000 × 109/L), in otherwise asymptomatic low-risk patients, a good reason to start cytotoxic therapy, nor do I adjust the dose of cytotoxic drugs in otherwise well-controlled high-risk patients only to normalize platelet count. Indeed, there is no evidence that thrombocytosis is a risk factor for thrombosis.69 Even though leukocytosis may be a risk factor for thrombosis, I do not correct moderate leukocytosis (<20 × 109/L) in a low-risk subject; however, progressive leukocytosis might require hydroxyurea and/or adjustment of the dose. In elderly subjects, busulfan could be particularly effective for extensive leukocytosis. If appropriate, allopurinol is prescribed for hyperuricemia.
I maintain all PV patients without contraindications on low-dose aspirin (100 mg daily); in case of gastric intolerance, I prefer to add anti-H2 medication rather than switching promptly to another antiaggregant agent. My attitude toward low-dose aspirin derives from results of the placebo-controlled European Collaboration on Low-Dose Aspirin in PV trial that demonstrated that the risk of the combined end point of nonfatal myocardial infarction, nonfatal stroke, pulmonary embolism, major venous thrombosis, or death from cardiovascular causes (relative risk, 0.40; 95% confidence interval, 0.18-0.91; P = .03) was significantly reduced by low-dose aspirin.44 However, the statistical significance of the findings has been questioned in a meta-analysis.70 Although there is preclinical evidence of better inhibition of platelet thromboxane production with aspirin twice daily,71 I do not use it routinely because safety has not been assessed. Acquired von Willebrand defect72,73 may underline a bleeding tendency, particularly in the presence of extreme thrombocytosis,74 and aspirin should be prescribed cautiously. Assessment of ristocetin cofactor activity may help in therapy decision,3 but in practice I do not use it.
Returning to our patients: prescribing therapy and follow-up
Case 1
The patient was classified as low risk. He had 13 phlebotomies performed in the first 3 months and then maintained an Hct <45% with 1 phelobotomy every 2 months for the next 2.5 years. The course was uneventful, the spleen remained nonpalpable, and baseline mild hypertension resolved spontaneously. He is on aspirin without side effects. He complains of increasing asthenia that limits some activities and can be largely ascribed to severe iron deficiency induced by phlebotomies. He is being followed up with biannual visits.
Case 2
The patient was considered high risk because of previous thrombosis and older age. I prescribed hydroxyurea that was maintained at a dose of 1.0 to 1.5 g daily depending on blood counts and aspirin. Phlebotomies were sporadic, no more than 2 to 3 per year. Eight years later, a marked enlargement of the spleen (10 cm from LCM) was discovered; there was leukocytosis (34.0 × 109/L with 5% myelocytes) and thrombocytosis (730 platelets × 109/L), and Hct was 44.2%. He only complained of fatigue. Hydroxyurea was increased to 2.0 g daily. However, he returned 4 months later complaining of weight loss (8% of body weight), night sweats, and abdominal discomfort. The spleen was now palpable at 13 cm. Blood tests showed the following: leukocytes, 37 × 109/L; leukoerythroblastosis with 3% blasts; platelets, 390 × 109/L; and Hb, 113 g/L. A BM biopsy showed diffuse grade 3 fibrosis. The JAK2V617F allele burden was 89%, and a del20q abnormality was found in 14 of 20 metaphases. A diagnosis of PPV-MF by the International Working Group for Neoplasms Research and Treatment criteria was made (Table 5).75-77 He was enrolled in a clinical trial with a JAK2 inhibitor and obtained marked reduction of spleen volume and improvement of symptoms; Hb decreased to a nadir of 91 g/L. He is still on therapy after 3 years with continuing benefits. The management of PPV-MF does not differ from PMF.78
Criteria for the diagnosis of PPV-MF by the IWG-MRT75
| . | Criteria . |
|---|---|
| Required criteria | Documentation of a previous diagnosis of PV as defined by the WHO criteria; and BM fibrosis grade 2 to 3 (on 0-3 scale) or grade 3 to 4 (on 0-4 scale)* |
| Additional criteria | Anemia† or sustained loss of requirement for either phlebotomy (in the absence of cytoreductive therapy) or cytoreductive treatment of erythrocytosis; a leukoerythroblastic peripheral blood picture; increasing splenomegaly, defined as either an increase in palpable splenomegaly of ≥5 cm (distance of tip of the spleen from LCM) or the appearance of a newly palpable splenomegaly; and development of ≥1 of 3 constitutional symptoms: >10% weight loss in 6 months, night sweats, and unexplained fever (>37.5°C) |
| . | Criteria . |
|---|---|
| Required criteria | Documentation of a previous diagnosis of PV as defined by the WHO criteria; and BM fibrosis grade 2 to 3 (on 0-3 scale) or grade 3 to 4 (on 0-4 scale)* |
| Additional criteria | Anemia† or sustained loss of requirement for either phlebotomy (in the absence of cytoreductive therapy) or cytoreductive treatment of erythrocytosis; a leukoerythroblastic peripheral blood picture; increasing splenomegaly, defined as either an increase in palpable splenomegaly of ≥5 cm (distance of tip of the spleen from LCM) or the appearance of a newly palpable splenomegaly; and development of ≥1 of 3 constitutional symptoms: >10% weight loss in 6 months, night sweats, and unexplained fever (>37.5°C) |
Diagnosis of PPV-MF requires ≥2 additional criteria beyond the 2 main criteria.
According to the European classification, grade 2 or 3 fibrosis is a diffuse, often coarse, network of fibers with no evidence of collagen formation by trichrome stain or a diffuse coarse fiber network with areas of collagen deposition stained positive by the trichrome staining.76 According to the standard classification, grade 3 or 4 fibrosis is a diffuse and dense increase in reticulin fibers with extensive intersections, with only occasional focal bundles of collagen and/or focal osteosclerosis or diffuse and dense increase in reticulin fibers with extensive intersections with coarse bundles of collagen, often associated with significant osteosclerosis.77
Below the reference range for appropriate age, gender, and altitude considerations.
Special situations of interest
Thrombosis in unusual sites
PV is a frequent underlying cause of SVT that includes Budd-Chiari syndrome (BCS), mesenteric, splenic, or portal vein thrombosis (PVT). Up to 70% of SVT is developed prior to MPN diagnosis.79 The estimated prevalence of MPN in patients with BCS and PVT is 30% to 50% and 15% to 30%, respectively.79 BCS is more frequent than PVT in PV.79 Young women are preferentially affected. Diagnosis of MPN-associated SVT is facilitated by the occurrence of JAK2V617F in 50% to 60% of BCS and 20% to 40% of PVT; conversely, peripheral blood counts may be minimally affected mainly because of hemodilution. Furthermore, BM biopsy may not be pathognomonic, and diagnosis of MPN subtypes may remain elusive. This is why, when I see a JAK2V617F-mutated woman with SVT, I keep in mind an instructive statement: “Until proven otherwise, an Hct of 45% in a woman with ascites, splenomegaly, and hepatic vein thrombosis suggests the presence of erythrocytosis masked by an expanded volume of plasma.”80 In selected patients, I order Cr-51 RCM to corroborate diagnosis. Inherited deficiencies of anticoagulant proteins, factor V Leiden, or prothrombin G20210A mutations should be evaluated.
Anticoagulation with low-molecular-weight heparin (LMWH) is instituted at the time of thrombosis discovery, followed by vitamin K antagonists. However, the duration of treatment is still a matter of debate81 ; the risk of bleeding in patients with portal hypertension and esophageal varices cannot be undermined. My policy is to maintain oral anticoagulation indefinitely because I consider the underlying PV as a permanent risk factor for rethrombosis. On the basis of the same reasoning, I prescribe cytotoxic therapy with hydroxyurea or interferon in younger women. I do not combine anticoagulation and aspirin, unless in selected cases, to avoid an excess of bleeding. The management of PV-associated SVT requires continuing collaboration with hepatologists.82
Resistance/refractoriness to first-line treatment
Over time, 10% to 15% of patients on hydroxyurea develop hematologic and extrahematologic (painful leg ulcers, skin cancers, or extensive dermatitis, and fever) toxicities86 and have to stop the drug or use a suboptimal dose (Table 687,88 ). These patients can be shifted to interferon-α, or busulfan if >65 years of age; those who do not tolerate first-line interferon should be put on hydroxyurea or busulfan. Busulfan is an alkylating agent that produces hematologic responses in most patients and a decrease of JAK2V617F allele burden in some89 ; it must be accurately titrated because of prolonged myelosuppression.90 I start at 2 mg daily and reduce promptly once the target level of Hct, leukocytes, and/or platelets is reached; maintenance dose may be a few milligrams per week, and many patients stop it after a while, yet maintain hematologic response. There is no firm evidence of an increased leukemogenic potential of busulfan, but studies are small and retrospective.91,92 Sometimes, a low-risk patient presents intolerance to phlebotomy or a too high requirement and must be shifted to cytotoxic therapy.
Criteria of clinical resistance and intolerance to hydroxyurea in PV based on the European LeukemiaNet consensus87
| Type . | Criteria . |
|---|---|
| Resistance | |
| 1 | Need for phlebotomy to keep Hct <45% after 3 months of ≥2 g/day of hydroxyurea, OR |
| 2 | Uncontrolled myeloproliferation, i.e., platelet count >400 × 109/L AND white blood cell count >10 × 109/L after 3 months of ≥2 g/day of hydroxyurea, OR |
| 3 | Failure to reduce massive* splenomegaly by more than 50% as measured by palpation, OR failure to completely relieve symptoms related to splenomegaly after 3 months of ≥2 g/day of hydroxyurea, OR |
| Intolerance | |
| 4 | Absolute neutrophil count <1.0 × 109/L OR platelet count <100 × 109/L or Hb <100 g/L at the lowest dose of hydroxyurea required to achieve a complete or partial clinico-hematological response†, OR |
| 5 | Presence of leg ulcers or other unacceptable hydroxyurea-related nonhematological toxicities, such as mucocutaneous manifestations, gastrointestinal symptoms, pneumonitis, or fever at any dose of hydroxyurea |
| Type . | Criteria . |
|---|---|
| Resistance | |
| 1 | Need for phlebotomy to keep Hct <45% after 3 months of ≥2 g/day of hydroxyurea, OR |
| 2 | Uncontrolled myeloproliferation, i.e., platelet count >400 × 109/L AND white blood cell count >10 × 109/L after 3 months of ≥2 g/day of hydroxyurea, OR |
| 3 | Failure to reduce massive* splenomegaly by more than 50% as measured by palpation, OR failure to completely relieve symptoms related to splenomegaly after 3 months of ≥2 g/day of hydroxyurea, OR |
| Intolerance | |
| 4 | Absolute neutrophil count <1.0 × 109/L OR platelet count <100 × 109/L or Hb <100 g/L at the lowest dose of hydroxyurea required to achieve a complete or partial clinico-hematological response†, OR |
| 5 | Presence of leg ulcers or other unacceptable hydroxyurea-related nonhematological toxicities, such as mucocutaneous manifestations, gastrointestinal symptoms, pneumonitis, or fever at any dose of hydroxyurea |
Organ extending by >10 cm from the LCM.
Complete response was defined as Hct <45% without phlebotomy, platelet count ≤400 × 109/L, white blood cell count ≤10 × 109/L, and no disease-related symptoms. Partial response was defined as: Hct <45% without phlebotomy, or response in three or more of the other criteria (European LeukemiaNet criteria).88
Pregnancy
I have followed some successful cases of pregnancy, although this event is definitely less common than in ET because of the older age of PV patients.93 In low-risk patients with no previous miscarriages, I suggest aspirin during the pregnancy and LMWH for 6 weeks after delivery. Hct is maintained at ≤40% with phlebotomies. In women with a history of severe pregnancy complications or thrombosis I use LMWH throughout pregnancy and consider interferon-α to control Hct or extreme thrombocytosis.94,95
Investigational agents
There has been a shortage of clinical trials with investigational agents in PV until very recently. Three classes of novel agents have been used in phase 2 to 3 studies: interferon, histone deacethylase (HDAC), and JAK1/2 inhibitors. As discussed above, pegylated interferons are often used as first-line therapy based on sound evidence from phase 2 studies of clinical and hematologic efficacy and unique activity against JAK2V617F-mutated cells, with some patients achieving durable complete molecular remission.63,66 A more rigorous positioning of interferons in the therapeutic armamentarium of PV must await the results of ongoing phase 3 studies: one comparing pegylated interferon α-2b with hydroxyurea (#NCT01259856, sponsored by the Myeloproliferative Disorders-Research Consortium) and the other comparing a novel monopegylated interferon-α 2b preparation (AOP2014) with hydroxyurea (#NCT01949805); both studies enroll PV patients who are naïve or currently treated with hydroxyurea. The HDAC inhibitor givinostat has been used in 2 phase 2 clinical trials: the first included patients with PV and other MPNs who were intolerant or refractory to hydroxyurea,96 and the second, envisioning the association of givinostat with low-dose hydroxyurea, enrolled PV patients who were unresponsive to the maximum tolerated dose of hydroxyurea.97 These studies provided initial evidence of efficacy regarding Hct and phlebotomy control and symptom improvement, with a good safety profile; However, no phase 3 study is planned to date. Another HDAC inhibitor, vorinostat, showed efficacy in normalizing blood cell counts and reducing pruritus and splenomegaly in newly diagnosed and previously treated PV patients who were in need of cytoreduction or were intolerant to other therapies, but it caused notable side effects, leading to a high discontinuation rate.98 On the basis of the favorable results of a phase 2 study in 34 patients who were refractory or intolerant to hydroxyurea,99 a phase 3 trial randomizing PV patients to the JAK1 and JAK2 inhibitor ruxolitinib (n = 110) vs best available therapy (n = 112) was designed: the RESPONSE trial. Final results have been presented recently, showing that a statistically significant greater proportion of patients receiving ruxolitinib obtained Hct control, reduction of phlebotomies, reduction of enlarged spleen, and improvement of total symptom score compared with best available therapy; of note, the rate of thrombotic events was lower in patients randomized to ruxolitinib (A.M.V., unpublished data). These results suggest that ruxolitinib may represent a new treatment option for PV patients who are resistant to, or unable to tolerate, hydroxyurea. As thoroughly discussed in a recent position paper, the “optimal therapy for PV can only be determined by the completion of randomized clinical trials”100 ; this is why I encourage participation in controlled studies that randomize high-risk patients or those resistant/refractory to conventional therapy.
Acknowledgments
The author thanks the many people that in different ways contributed to this work with teaching, discussion, and experience sharing. These include my younger, passionate, and tireless colleagues and fellows in Florence. Tiziano Barbui fascinated me with these disorders many years ago, and the author feels particularly fortunate for his continuous scientific support; the author would like to dedicate this manuscript to him. Ayalew Tefferi has been a reference point and strong promoter of new ideas and projects in the last years. Conversely, the author apologizes to many colleagues whose work could not be cited here because of space constraints, yet contributed significantly to the field; the author is proud of the scientific interactions and friendship with many of them. The author is indebted to the many patients who made me understand how PV impacts on their life and donated their blood for research. Finally, the author acknowledges the support from Associazione Italiana per la Ricerca sul Cancro through the fund “AIRC 5 per Mille” (#1005) to the AIRC-Gruppo Italiano Malattie Mieloproliferative (www.progettoagimm.it) group. Without such a support, many of the studies performed in Italy and cited in this manuscript would not have been possible.
Authorship
Contribution: A.M.V. wrote the manuscript.
Conflict-of-interest disclosure: A.M.V. receives advisory board and lecture honoraria and institutional research support from Novartis.
Correspondence: Alessandro M. Vannucchi, Laboratorio Congiunto Malattie Mieloproliferative Croniche, Department of Experimental and Clinical Medicine, University of Florence, Largo Brambilla 3, 50134 Florence, Italy; e-mail: amvannucchi@unifi.it.