Abstract
Introduction:
The outcome of BCR–ABL positive acute lymphoblastic leukemia (ALL) has drastically improved since the introduction of imatinib. We recently reported the clinical results of the Japan Adult Leukemia Study Group (JALSG) ALL-202 study at the 51th ASH Annual Meeting. Most patients (97.1%) achieved complete remission (CR) and 9% of them relapsed within 100 days after CR. In addition, 60% of patients received allogeneic stem cell transplantation during their first CR, and the 3-year overall survival (OS) rate was 57%. We now present the data of the subsequent JALSG Ph+ALL208 study, where we have modified a part of consolidation therapy to prevent early relapse after achieving CR.
Methods:
The JALSG Ph+ALL208 study was a phase 2 trial for patients newly diagnosed with BCR–ABL positive ALL. Imatinib at a dose of 600 mg/day was administered from day 8 to day 42 combined with daunorubicine (DNR), cyclophosphamide (CPM), vincristine (VCR) and prednisolone (PSL) for induction therapy. Consolidation therapy comprised course 1 (C1: high-dose methotrexate and high-dose cytarabine with imatinib for 18 days) and course 2 (C2: DNR, CPM, VCR, and PSL with imatinib for 20 days). C1 and C2 were repeated alternately for 4 cycles. After consolidation therapy, allogeneic stem cell transplantation (allo-SCT) was recommended if a suitable stem cell donor was identified. Those ineligible for allo-SCT, due to the lack of a suitable donor and/or comorbidity, received maintenance therapy comprising VCR, PSL and imatinib for 2 years from the date they achieved CR. Seventy patients were enrolled between October 2008 and December 2010. Of these, two patients were excluded because they were diagnosed with chronic myeloid leukemia blast phase. Therefore, 68 patients newly diagnosed with BCR–ABL positive ALL were included in this study. The median age was 49 years (18–64 years) and 41% were >54 years.
Results:
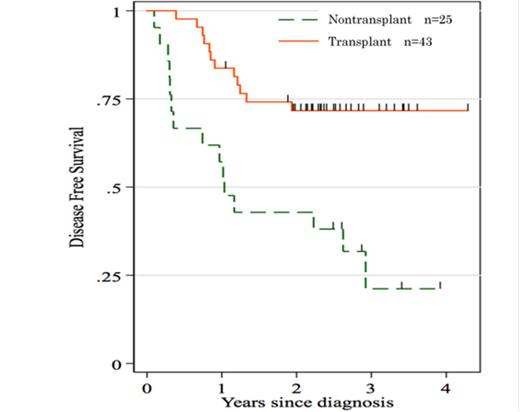
With this treatment regimen, 65 patients achieved CR (95.6%) and only 1% of them relapsed within 100 days after CR. Finally, 35/40 patients (81%) <55 years-old="" and="" 8="" 28="" 19="">54 years-old were able to receive allo-SCT in their first CR (13 from a sibling donor, 23 from an unrelated bone marrow donor, and 7 from unrelated cord blood). The 3-year OS and disease-free survival (DFS) rates were estimated at 62% and 52%, respectively. Three early deaths occurred during the induction course. One patient (51 years) died of pulmonary bleeding on day 9, another patient (59 years) died of sepsis on day 15, and the third patient (62 years) died of cerebral hemorrhage on day 15. Grade 3 or 4 non-hematologic adverse events including febrile neutropenia and liver dysfunction was reported in 60.3% and 11.8% of the patients, respectively. Twelve patients relapsed after achieving CR with a median time of 307 (64–1053) days. Moreover, 6/43 patients who received allo-SCT relapsed with a median time of 346 (149–602) days. The probability of DFS at 3 years was 72% for patients who underwent allo-SCT in CR compared to only 21% for patients without allo-SCT (p = 0.0004)(Figure.1).
Conclusion:
We conclude that imatinib-based chemotherapy produced a very high CR rate, thus allowing a high proportion of patients to prepare for allo-SCT, particularly patients 55 years. Moreover, the intensified consolidation therapy reduced the rate of early relapse after induction therapy and resulted in a higher rate of DFS after allo-SCT.
Hatta:Bristol Myers Squibb: Honoraria. Miyazaki:Novartis: Honoraria. Ohnishi:Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal