Abstract
Introduction: The FLT3-ITD mutation is associated with a high relapse rate for patients with AML even after allogeneic hematopoietic stem cell transplantation (HSCT). Sorafenib is an oral tyrosine kinase inhibitor which inhibits the FLT3 tyrosine kinase. Evidence based on FLT3-ligand levels has suggested that the maintenance setting may be the optimal time to administer FLT3 inhibition.
Methods: We conducted a phase I trial of maintenance sorafenib after HSCT in patients with FLT3-ITD AML. Patients received a variety of conditioning regimens (12 myeloablative, 10 reduced intensity) and graft sources (19 matched related / unrelated, 1 haploidentical, 1 double umbilical cord blood, 1 mismatched unrelated). All patients were in morphological remission with predominant donor chimerism after HSCT before starting sorafenib. A dose escalation 3+3 cohort design was used to define the maximum tolerated dose (MTD) with an additional 10 patients treated at the MTD. Sorafenib was initiated between days 45 and 120 after HSCT and given continuously for twelve 28-day cycles. The DLT period was the first cycle of treatment.
Results: Twenty-two patients with FLT3-ITD AML were enrolled (status at HSCT: CR1=16, CR2=3, refractory=3). The MTD was established at 400 mg BID with one DLT observed (pericardial effusion) which was later deemed unrelated to sorafenib. Two patients died of transplant-related causes and neither was deemed related to sorafenib (idiopathic pneumonia syndrome, cirrhosis from iron overload). Two patients stopped sorafenib after relapse and 5 stopped sorafenib due to attributable toxicities after the DLT period. The most common toxicities observed were skin and GI with dose reductions and interruptions common. There were no significant flares of acute GVHD observed after starting sorafenib and 1-year cumulative incidence of chronic GVHD was 42% (90% CI, 23%, 60%). Effective inhibition of FLT3 as measured by a plasma inhibitory assay was observed at all doses employed. Serial levels of FLT3 ligand were measure in 7 patients. Median level at baseline and prior to any sorafenib administration was 125 pg/ml (range 40-323) and this significantly increased to a median level of 254 pg/ml (range 80-500) (p = 0.016) measured on day 29 of cycle 1.
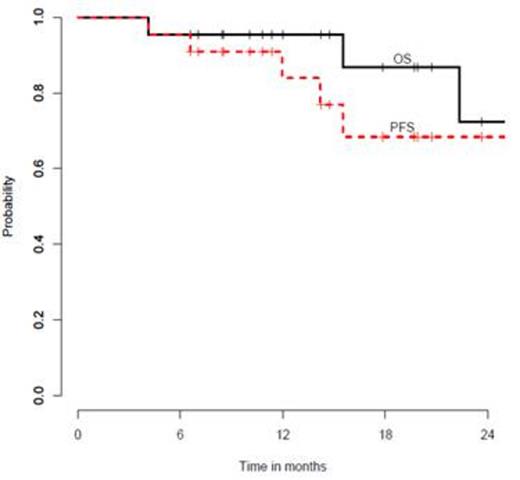
The median follow-up for survivors is 14.5 months (range, 6.6-34.0) after HSCT. One-year progression-free survival is 84% (90% CI, 63%-94%) and one-year overall survival is 95% (90% CI, 79%-99%) (Figure 1). Only one patient who underwent HSCT in CR (n=19) has relapsed.
Conclusion: Sorafenib is safe to give as maintenance therapy after HSCT for patients with FLT3-ITD AML and may reduce the rate of relapse. Maintenance sorafenib or other FLT3 inhibitors after HSCT merits further investigation.
Chen:Bayer Pharmaceuticals, Inc.: Other, Research Funding. Off Label Use: Sorafenib as maintenance therapy for FLT3-ITD AML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal