Abstract
Antiviral therapy (interferon alpha with ribavirin) for chronic hepatitis C virus infection (c-HCVI) frequently causes decrease of cell blood counts (CBC) which may affect undertaken treatment. The aim of the study was to evaluate the frequency of CBC changes during antiviral therapy and to assess if pre-treatment CBC declines affect the incidence and severity of CBC alterations.
This retrospective analysis included 618 patients, diagnosed with c-HCVI in the years 1998-2010 at the Pomeranian Region, Poland treated with interferon alpha +/- ribavarin. The data were collected at the following time points: start of treatment (week 0) and at the end of 12-th, 24-th and 48-th week of treatment. Hemoglobin (HGB), leukocyte count (WBC) absolute neutrophil count (ANC) and platelet count (PLT) declines were graded as mild//moderate if HGB was not lower than 10//8 g/dl, WBC than 3//2 G/l, ANC than 1//0.75 G/l and PLT than 100//50 G/l. To determine the clinical significance of the observed CBC changes, values: HGB<10 g/dl, ANC <0.75 G/l, PLT <50G/l listed in the Summary of Product Characteristics Pegasys (peginterferon alpha-2a) as the dose reduction and stop-treatment values were used as cut-off values. Data were analyzed using Wilcoxon test.
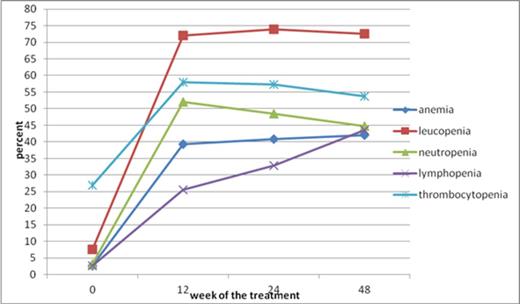
During the first 12-th weeks of antiviral therapy a statistically significant decrease (p<0.05) in HGB, WBC, ANC, lymphocyte count (ALC) and PLT was observed leading to the statistically significant increase in the frequency of anemia (HGB≤11.5 g/dl), leucopenia (WBC<4 G/l), neutropenia (ANC<1.5 G/l), lymphopenia (ALC<1 G/l) and thrombocytopenia (PLT<150 G/l) at week 12. The most common abnormality was leucopenia that occurred in 78.4% of women and 67.8% of men. In the majority of patients (about 94%) CBC declines were mild or moderate. Taking into account the dose reduction and stop-treatment values CBC declines exceeded at least one of them in 17.5% of patients. The most frequent decline was observed in ANC: 8% of patients had clinically significant ANC decrease. During the therapy the frequency and severity of CBC changes didn't change with the exception of lymphopenia that progressively increased from 29.1% to 50.4% in women and from 21.2% to 39.6% in men at 48-th week (Figure 1) .
The incidence of CBC abnormalities during antiviral treatment in patients with c-HCVI.
The incidence of CBC abnormalities during antiviral treatment in patients with c-HCVI.
To determine, if pre-treatment CBC abnormalities affected the CBC results during therapy, we compared the patients with and without CBC pre-treatment declines. Patients with normal CBC before treatment had statistically significant decrease in the HGB concentration, WBC, ANC, ALC and PLT at the 24-th and 48-th week of the treatment. Among patients with pre-treatment CBC declines only patients with leucopenia and thrombocytopenia had a statistically significant decrease in WBC and PLT numbers at the 24-th and 48-th week of treatment. Clinically significant decrease in CBC during antiviral therapy was more frequent in patients who had reduced CBC before the therapy. The most frequent clinically significant decrease was observed in ANC at the 12-th and 24-th week of the treatment in respectively 43% and 41% of patients with neutropenia before the treatment. The clinically significant decrease in the HGB and PLT at the 12-th and 24-th week of the treatment was observed in respectively 33%, 29% and 8.6%, 7.5% of patients with anemia and thrombocytopenia before the treatment. We conclude that CBC declines in patients treated for c-HCVI occur frequently, but remain mild and moderate. The number of patients with clinically significant declines in CBC (mainly ANC) was very small. CBC falls occurred more often in patients with pre-treatment reduced CBC, however in most of the patients the CBC declines did not reach clinical significant CBC values. To conclude, reduced CBC before the treatment should not preclude antiviral treatment with interferon alpha +/- ribavirin though clinically significant declines occur more often in these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal