Abstract
The combination of vorinostat, a histone deacetylase (HDAC) inhibitor, is synergistic with proteasome inhibitors and IMIDs based upon preclinical and clinical data. Phase III trials with vorinostat in relapsed myeloma failed to demonstrate benefit due to excess toxicity that precluded chronic administration of the agent allowing patients to realize the clinical benefits. Herein we report on our experience combining vorinostat, or adding vorinostat to current treatments in the context of refractory myeloma.
Methods: Forty RRMM pts that received vorinostat (V) (200mg orally daily on days 1-14) and bortezomib (B) (1.3 mg/m2 IV/SQ days 1,4,8, and 11) combinations, every 28 days from May 2008 until May 2014 at Emory University were reviewed. Cohort 1 consisted of 22 RRMM pts that received VBCyTD regimen (V,B, cyclophosphamide (Cy) (300 mg orally flat dose days 1, 8 and 15), thalidomide (T) (100 mg daily) and dexamethasone (D) 40 mg orally weekly), while cohort 2 consisted of 18 RRMM pts that received other combinations (VBCyD+lenalidomide (R): 4 pts; VBRD: 4 pts; VBCyD: 2 pts; VBD: 4 pts; VBTD: 4 pts and VCyTD+carfilzomib (Ca): 2 pts). The objectives are response assessment by IMWG criteria, progression-free survival (PFS) and overall survival (OS). Drug related adverse events (AEs) ≥grade 3 (CTCAE v3.0) were summarized.
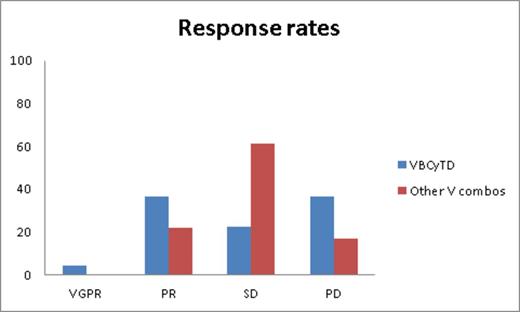
Results: Twenty-one males and nineteen females (median age at diagnosis: 56 years [range 28-89], median age at initiation of V treatment: 62 years [range 33-91]) that were heavily pretreated (median lines of therapy: 7 [range 3-14] were included in the analysis. Ninety-five percent of pts were B refractory and 85% pts were R refractory; 97.5% and 15% of pts underwent prior autologous and allogeneic SCT, respectively. High risk cytogenetic abnormalities were seen in 40% of pts. Time from diagnosis to initiation of V combination therapy is 58.5 months (range 18-138). The overall clinical benefit rate (≥SD) was 72.5%. Figure 1 illustrates response rates from both cohorts. Median PFS for cohort 1 is 3 months (95% CI 0.5-5.51) and for cohort 2 is 2 months (95% CI 0.8-3.2), respectively. At a median follow-up of 26 months from the time of V initiation, median OS for cohort 1 is 6 months (95% CI 0-13.6) and for cohort 2 is 3 months (95% CI 1.4-4.7). Median overall survival from diagnosis for these cohorts is 80 months (95% CI 70.2-89.8) and 70 (95% CI 55.6-84.4) months, respectively. ≥Grade 3 AEs include fatigue (15%); generalized weakness (12.5%); neutropenia (15%); and thrombocytopenia (35%).
Conclusions: In heavily pretreated relapsed/refractory multiple myeloma patients with limited therapeutic options, vorinostat and bortezomib combination therapy offers clinical benefit with good response rates and acceptable tolerability, likely due to lower doses of vorinostat used. This data further supports the activity of HDAC inhibitors in the context of refractory myeloma.
Gleason:Celgene: Consultancy; Novartis: Consultancy. Heffner:Amgen: Honoraria, Research Funding; Biotest: Honoraria, Research Funding; Dana Farber CI: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Idera: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; Onyx: Honoraria, Research Funding; Spectrum: Honoraria, Research Funding; Talon Therapeutics: Honoraria, Research Funding. Lonial:Millennium: The Takeda Oncology Company: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Onyx Pharmaceuticals: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal