Abstract
Introduction: Gene expression profiling (GEP)-defined high-risk de novo multiple myeloma (HI-MM) has a dismal prognosis with median PFS and OS stagnating at 2 and 3 years, respectively, despite the incorporation of novel agents into our Total Therapy (TT) trials. Having seen encouraging results in relapsed-refractory MM with an extended 16-day metronomic therapy (Papanikolaou, Haematologica 2014), we tested this approach in a small cohort of untreated patients with HI-MM. METRO emphasizes targeting neo-angiogenesis and other components of the bone marrow micro-environment while avoiding cytokine surges with recovering hematopoiesis following myelotoxic therapy.
Patients and Methods: 10 previously untreated patients with HI-MM, who were either ineligible or unwilling for our Total Therapy protocols received a single cycle of METRO. Therapy comprised of SC bortezomib 1.0mg/m2 (0.8mg/m2 in case of grade >2 peripheral neuropathy) on days 1, 4, 7, 10, 13, 16, 19, 22, 25 and 28 schedule, PO dexamethasone 12mg (8mg in case of prior intolerance of higher dose or diabetes mellitus) on days 1 to 4, 7 to 10, 13 to 16, 19 to 22 and 25 to 28, PO Thalidomide 100mg (50mg in case of peripheral neuropathy grade >2) and continuous IV infusions of doxorubicin and cisplatin at 1.0mg/m2 daily for 28 days. Cisplatin was dose-reduced for Cr >2mg/dL and omitted for Cr >3mg/dL. Arsenic tri-oxide was given at a fixed dose of 0.01mg/kg on the days after bortezomib. Laboratory monitoring for response and toxicities were done on a Monday-Wednesday-Friday schedule. Maximal responses, based on current IMWG definitions, were measured within 30 days of completion of cycle 1, and at least monthly thereafter. KM curves were current as of 07/31/14. The Institutional Review Board granted permission for our retrospective data review, the results of which are presented here.
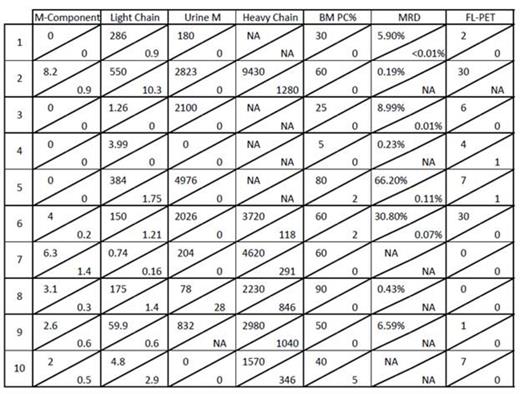
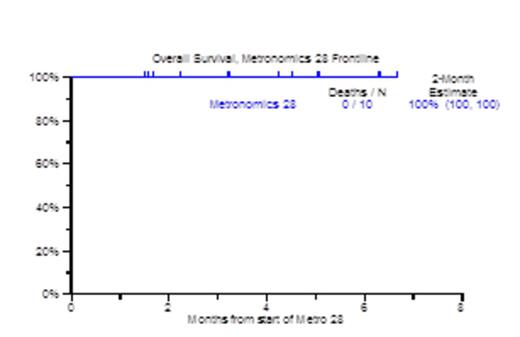
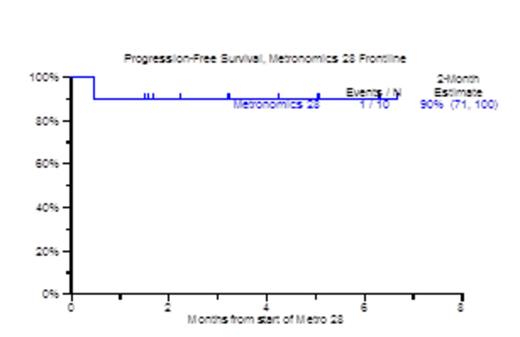
Results: Patient characteristics included age >=65 in 8, 5 male, 5 female with ISS III in 5 patients. Metaphase cytogenetic abnormalities (CA) were detected in 7 patients. GEP70 based high risk MM was present in all 10 patients, and GEP proliferation (PR) subgroup was dominant in 8 out of 10 patients. All 10 patients achieved at least PR, including 3 qualifying for VGPR and 4 for CR. Bone Marrow responses were equally encouraging in that 8 of 10 patients qualified for complete morphologic negativity including 4 with no minimal residual disease (MRD) by 8-color flow cytometry. Of 8 patients with FDG-avid PET-CT focal lesions, 6 achieved PET-CT CR; all patients showed decreases in SUV-max and SUV-diff (background SUV). Number and/or apparent diffusion coefficient (ADC) mapping of focal lesions and background marrow on diffusion-weighted MRI improved in all 7 evaluable patients. GEP70 risk morphed from high risk to low risk in 3/4 evaluable patients. Pre and post serologic, urinary and radiologic responses are shown in Figure 1. The median follow-up time for the population was 3.2 months (98 days). All 10 patients are alive from 1 to 7 months, and 1 suffered progression (Figure 2). Overall tolerance was good. Non-hematologic grade 3/4 SEs included fatigue, electrolyte abnormalities (20%), dyspnea, hypotension, LE edema and transaminitis (10%).
Conclusion: Primary 28-day metronomic therapy is highly effective and well-tolerated in patients with previously untreated HI-MM. Further prospective studies with longer follow-up are currently being devised in an attempt to improve outcomes in this population.
Individual responses
van Rhee:Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Zangari:Norvartis: Membership on an entity's Board of Directors or advisory committees; Onyx: Research Funding; Millennium: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal