Abstract
Despite a reported increased incidence of secondary hematologic malignancies and plasma cell disorders in the literature, the specific co-existence of hairy cell leukemia (HCL) and monoclonal gammopathies of unknown significance (MGUS) or plasma cell multiple myeloma (MM) has been based on a few case reports. In the following report, we compile clinico-pathologic data on a series of hairy cell leukemia patients with myeloma precursor diseases using immunohistochemistry, molecular polymerase chain reaction (PCR) and multi-parametric flow cytometry (MFC) techniques with an aim to deep sequence these parallel lymphoid processes in the future.
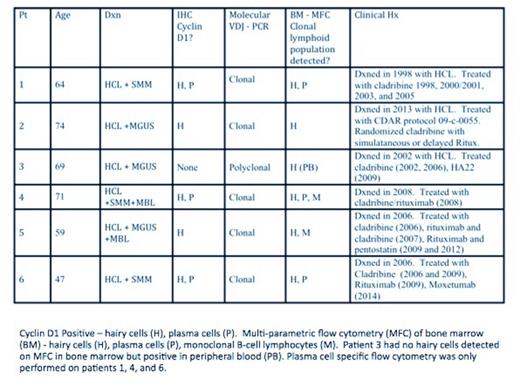
Between 2004 and 2014, 6 HCL patients followed at the National Institutes of Health were identified with associated plasma cell disorders, based on presence of increased clonal plasmacytosis in bone marrow and abnormal serum and/or urine protein electrophoresis/immunofixation. Immunohistochemical (IHC) staining for CD20, TRAP, CD138, cyclin D1, kappa and lambda light chains was performed on bone marrow biopsies. MFC using a panel of B-cell antibodies and PCR clonality studies targeting immunoglobulin heavy and light chain loci were performed prior to treatment to confirm the presence of malignant lymphoid clonal processes.
Among the 6 hairy cell leukemia patients with associated plasma cell disorders, there were 5 males and one female. Median age was 67 (range, 47-74). Patients prior to treatment showed bone marrow involvement by HCL with 5-90% infiltration. Hairy cells in all patients tested were TRAP positive, Cyclin D1 was positive in 5, and BRAF V600E was detected in 5 of 5 patients tested. All patients were diagnosed as classic HCL, although 1 patient had an additional population consistent with HCL variant. In addition, 3/6 patients (50%) showed mild increase in marrow plasma cells (5-10%) and the other 3 patients (50%) had over 10% of plasma cells in the core biopsy. The mean plasma cell percentage was 10% (3-25%), mean monoclonal protein concentration was 1.3 g/dL, and isotypes included: 4 IgG, 1 IgA, and 1 free kappa only. 3 patients were classified as MGUS and 3 as smoldering MM (SMM). Interestingly, three out of 6 (50%) patients had positive cyclin D1 expression by IHC in both plasma cells and hairy cells. Several patients had evidence of multiple clonal rearrangements by PCR studies. In addition, two patients demonstrated evidence of monoclonal B-cell lymphocytosis (MBL) on MFC. So far, 3 patients achieved complete remission without minimal residual disease (MRD) using moxetumomab pasudotox in one multiply relapsed case, and first line cladribine plus rituximab in 2 newly diagnosed HCL cases, without significant progression to MM. No patients demonstrated end organ damage due to MGUS/SMM after a median follow-up of 4.6 (range 0.9-10.1) years.
By using serum studies, IHC staining, PCR and MFC tools, we identified a group of HCL patients with evidence of additional precursor malignant lymphoid disease states, including MGUS/SMM and MBL. Underlying mechanisms of these parallel malignant processes may include global lymphoid dysregulation through common lymphocyte ancestry pathways; or, it could be due to post-immune HCL therapy exposure, or a combination. Currently, we are conducting deep sequencing of these samples with the aim to uncover mechanisms of pathogenesis. Treatments capable of eliminating HCL MRD, including addition of rituximab to first-line cladribine, or single-agent moxetumomab pasudotox for multiply relapsed HCL, might be advantageous for patients who may need treatment for MM in the future.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal