Abstract
Background : Low riskMyelodysplastic Syndrome (LR-MDS) is a cytogenetic, epigenetic and immunologically heterogeneous group of disorders associated with refractory anemia (RA) in about 75% of the cases. Conventionally, blood transfusion and Erythropoiesis Stimulating Agents (ESAs) are indicated. Azanucleosides provide clinical benefit in about 20-25% of patients (pts) with ESA refractory disease highlighting the need for new drug development. Chronic inflammation derived from deregulated cytokines with myelosupressive potential, ( IL6, TNF-ɑ and INF-λ) is an important factor in LR-MDS. Selective-Serotonin-Reuptake-Inhibitors (SSRIs) provide clinical benefit in depressed and rheumatoid arthritis pts, at least in part by stabilizing TNF-ɑ and IL6 levels (Brunoni AR., et al. Ann Rev of Med. 2013). In colon and prostate cell lines SSRIs inhibit proliferation. This data suggests that SSRIs may act in at least two ways that would be beneficial in MDS. Here we investigate clinical benefit of SSRIs in treating a subgroup of MDS patients with refractory anemia (RA-MDS).
Methods: One hundred and twenty patients over the age of 18-diagnosed with MDS were identified in the Michael E. DeBakey VA Medical Center cancer registry between January 1, 2000 to December 31 2012. Of these, 55 LR-MDS pts with anemia were identified. Patients were considered to be SSRI+ if they had received at least 6 months of a SSRI (including fluoxetine, sertraline, paroxetine or citalopram) in the 12 months preceding or following the diagnosis of MDS. Overall Survival for the SSRI+ and SSRI- groups were estimated by the Kaplan-Meier method. Multivariate Cox regression analysis was performed to assess the impact of multiple independent variables on clinical outcome
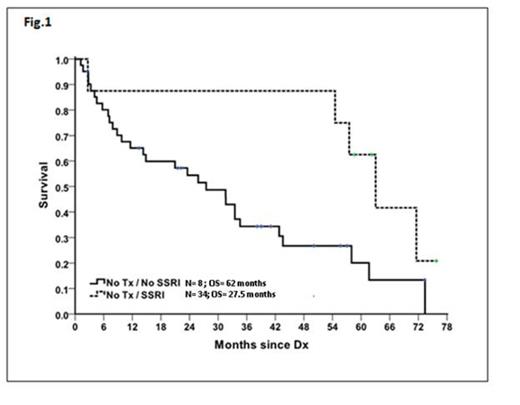
Results: Among the 55 patients studied, 8 and 47 belonged to SSRI+ and SSRI- groups, respectively. Mean age in the SSRI+ group was 69.5 years (median 72, range 55 to 82) and in the SSRI- group was 74.2 years (median 75, rangе 53 to 88); P=0.252. WHO 2008 classification for SSRI+ group was RUCD 3 [37.5%] and RCMD 5 [61.5%]; For the SSRI- group the classification was RUCD 13 [27.7%]; RCMD 30[63.8%]; RAEB 2[4.3]; MDS MPN unclassified 1[2.1%] and MDS 5q- 1[2.1%]. Median hemoglobin (Hb) level in the SSRI+ group was 10.5 g/dL (8-12 g/dL) and in the SSRI- group was 8.9 g/dL (range, 6-13), P=0.027. Given the central interest of anemia impact on Overall Survival (OS), adjustment for heterogeneity in Hb levels among SSRI+ and SSRI- groups was performed using subgroup survival analysis in pts with equivalent Hb levels (pts with Hb<8 were excluded resulting in P=0.067). Median OS was 62 months (range, 15.3-73.7) for the SSRI+ group (N=8) and 27.5 months (range, 15.7-39.3) for SSRI- pts (N=34) (P=0.0323, HR=3.045, 95% CI=[1.155,8.028]) (Fig. 1). Blast count, cytogenetic group and R-IPSS score did not impact OS on univariate or multivariate analysis.
Conclusions:Improved survival of LR-MDS anemic pts is central to treatment paradigms of MDS. Our study suggests survival improvement in LR- MDS pts treated with SSRIs. Potential clinical beneficial effect of SSRIs could be restricted to LR-MDS pts presenting with anemia. Prospective studies aimed at investigating molecular mechanisms of action, potential overall response rate as single agents or in combination with other MDS directed therapy are needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal