Abstract
Preclinical studies previously reported by our group at ASH suggest that treatment with granulocyte-stimulating factor (G-CSF) may provide a potent and well-tolerated method to disrupt the 'leukemia cell niche'. Specifically, our data show that G-CSF treatment in mice reprograms bone marrow stromal cells, markedly inhibiting their expression of key chemokines/cytokines that support B lymphopoiesis, including stromal derived factor-1 (SDF-1/ CXCL12), interleukin-6 (IL-6), insulin-like growth factor-1 (IGF-1), and interleukin-7 (IL-7). These changes in the bone marrow microenvironment result in a more than 10-fold decrease in B cells in the bone marrow, including early B cell precursors. Preliminary studies in humans suggest that G-CSF has a similar effect on the bone marrow microenvironment in healthy subjects. Since these B trophic factors also have been implicated in the maintenance of B cell acute lymphoblastic leukemia (ALL) cells, we hypothesize that G-CSF treatment in patients with acute lymphoblastic leukemia (ALL) will generate a 'hostile' bone marrow microenvironment for ALL cells, depriving them of key support signals and rendering them more sensitive to cytotoxic therapy.
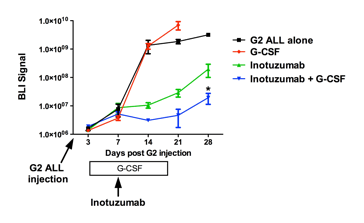
Herein, we specifically test whether niche disruption with G-CSF augments inotuzumab-mediated ALL cell clearance. Inotuzumab ozogamicin (Pfizer) is a CD22-calicheamicin antibody-drug conjugate. Initial clinical trials have documented activity of inotuzumab in B-ALL, with an overall response rate of 57% in relapsed or refractory B-ALL. To test this hypothesis, we developed a xenotransplantation model of human ALL using the human B-ALL G2 cell line, which expresses moderate levels of CD22. G2 cells transduced with a GFP-click beetle red luciferase retroviral construct to allow for in vivo bioluminescent imaging were injected intravenously into NOD/SCID/γc-/- (NSG) mice. The G2 cells preferentially home to the bone marrow but eventually metastasize to other organs, including the brain. Four cohorts of mice were studied (n = 9-21, each), including: 1) G2 ALL cells alone; 2) inotuzumab alone (single injection of 80 µg/kg, IP); 3) G-CSF alone (100 µg/kg/day for 14 days by continuous subcutaneous infusion); or 4) G-CSF plus inotuzumab (G-CSF was started four days prior to inotuzumab). Treatment with inotuzumab increased median survival from 25.5 days to 41 days (P < 0.0001). G-CSF alone had no effect on survival. However, combination of G-CSF plus inotuzumab further increased survival to 49 days (P = 0.01 compared with inotuzumab alone). G-CSF also improved survival in mice given two doses of inotuzumab (each 80 µg/kg, IP) 10 days apart. Bioluminescent imaging revealed that, in several of the mice in the G-CSF plus inotuzumab group, the bioluminescent signal was predominantly localized to the brain, with little or no signal in bone.
These data are consistent with the possibility that inotuzumab may have limited penetration into the brain. Thus, the positive effect of combination G-CSF plus inotuzumab on survival may be limited by CNS disease in this model. Indeed, the addition of G-CSF to inotuzumab decreased the bioluminescent signal in bone regions on day 21 after inotuzumab by approximately 10-fold (Figure 1, P < 0.001). Collectively, these data suggest that G-CSF significantly augments inotuzumab-mediated ALL clearance from the bone marrow. G-CSF may provide a non-toxic and cost-effective approach to increase durable response rates to inotuzumab (and other types of immunotherapy) in patients with B ALL.
Link:Pfizer: provided Inotuzumab for preclinical studies Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal