Abstract
The use of Bcr-Abl tyrosine kinase inhibitors (TKIs) has led to excellent clinical responses in patients with chronic phase chronic myeloid leukaemia (CML). However these TKIs have been less effective as single agents in blast phase (BP) CML and this represents an urgent unmet need. A number of novel agents are now being investigated but most have not been translated to the clinics yet. Pyrvinium, a FDA-approved anthelminthic drug, was reported to selectively inhibit growth of a number of tumour cell types including myeloma and erythroleukaemia. We investigated the effect and mechanism of action of pyrvinium in BP-CML.
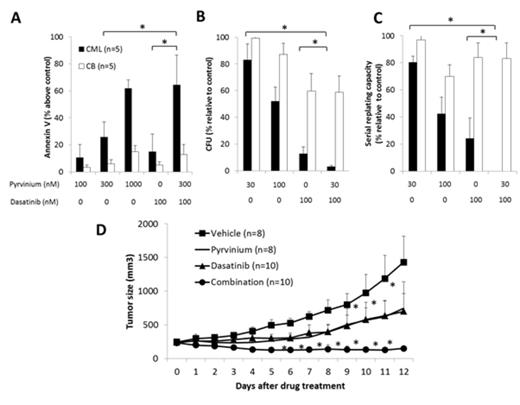
Our results show that pyrvinium selectively targeted BP-CML CD34+ progenitor cells. It induced apoptosis in CD34+ cells from TKI-resistant BP-CML patients who harbour Bcr-Abl kinase mutations, while sparing normal cord blood CD34+ cells. In addition, pyrvinium was more effective in inhibiting colony formation and self-renewal capacity in BP-CML CD34+ cells than cord blood CD34+ cells. Further increase in apoptosis, decrease in colony formation and self-renewal were seen when dasatinib was combined with pyrvinium in BP-CML but not cord blood CD34+ cells (Figure 1 A-C). We also showed that pyrvinium was synergistic in combination with dasatinib in inhibiting proliferation and inducing apoptosis in CML cell lines. We next tested the effects of pyrvinium and its combination with dasatinib in a CML xenograft model and showed that pyrvinium significantly delayed tumour growth with no signs of toxicity in the mice. When combined with dasatinib, the tumour growth was completely inhibited (Figure 1D). These results indicate that pyrvinium is active in BP-CML in vitro and in vivo.
The anti-cancer effects of pyrvinium have been reported to due to allosteric activation of casein kinase 1α (CK1α) and suppression of Wnt/β-catenin signalling (Thorne et al, Nature Chemical Biology 2010). However we showed that despite the efficient knockdown of CK1α and overexpression of β-catenin, the proliferation inhibitory effect of pyrvinium was not abolished, indicating a CK1α and β-catenin-independent mechanism of action in BP-CML. Instead, we observed that pyrvinium preferentially localized to mitochondria in CML cells and that pyrvinium inhibited oxygen consumption of CML cells within 5 minutes of treatment. In mitochondrial respiratory chain-deficient CML ρ0 cells which lack mitochondrial DNA and have undetectable oxygen consumption, we showed that the effect of pyrvinium in reducing ATP levels and inducing apoptosis was abolished. These results indicate that pyrvinium acts in CML through the inhibition of mitochondrial respiration.
We have shown that pyrvinium alone and in combination with Bcr-Abl TKI selectively targets BP-CML progenitor cells and pyrvinium acts in BP-CML through the inhibition of mitochondrial respiration. Given that pyrvinium is already clinically available, our pre-clinical findings can be translated rapidly into the clinics. Our data also suggests that targeting mitochondrial respiration may be a potential therapeutic strategy in aggressive leukaemia.
(A) Pyrvinium induces apoptosis of BP-CML CD34+ but not CB CD34+ cells and combination of pyrvinium and dasatinib is superior in inducing apoptosis. Pyrvinium is more effective in decreasing colony formation (B) and serial replating capacity (C) in BP-CML CD34+ than CB CD34+ cells. The combination is significantly more effective than single drug treatment (* p<0.01). (D) Pyrvinium is effective in vivo and significantly enhances the tumour growth inhibition of dasatinib (* p<0.01).
(A) Pyrvinium induces apoptosis of BP-CML CD34+ but not CB CD34+ cells and combination of pyrvinium and dasatinib is superior in inducing apoptosis. Pyrvinium is more effective in decreasing colony formation (B) and serial replating capacity (C) in BP-CML CD34+ than CB CD34+ cells. The combination is significantly more effective than single drug treatment (* p<0.01). (D) Pyrvinium is effective in vivo and significantly enhances the tumour growth inhibition of dasatinib (* p<0.01).
Off Label Use: The use of pyrvinium in leukaemia. Chuah:Novartis: Honoraria; Bristol-Myers Squibb: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal