Abstract
Background
Spontaneous loss of RHD expression is unusual and may present on routine RHD typing with new “mixed field” (separate positive and negative subpopulation) reactions. Its detection has practical and mechanistic implications for underlying pathology, and such events have been associated with the development or progression of malignant disorders.
Case
A 49-year-old gentleman with Acute Myelogenous Leukemia (AML) underwent standard induction chemotherapy with cytarabine and daunorubicin, achieving a remission. He moved from Saudi Arabia to Canada and presented to our center with an infected central venous catheter, whereupon a repeat marrow was performed, confirming remission (myeloblasts <5%) without peripheral blood count recovery. Review of the original diagnostic bone marrow revealed a predominance of promonocytes and monocytes, raising suspicions of pre-existing chronic myelomonocytic leukemia (CMML), while the presence of splenomegaly prompted investigation for a myeloproliferative neoplasm, wherein JAK2 and BCR-ABL testing ultimately proved negative. No cytogenetic abnormalities were noted at diagnosis or post-induction.
His presenting transfusion laboratory sample typed as O, RHD-positive with a negative red cell antibody screen, and stable repeat grouping for two months. Platelet transfusion refractoriness developed (PRA 99%), and he qualified for HLA-matched platelets.
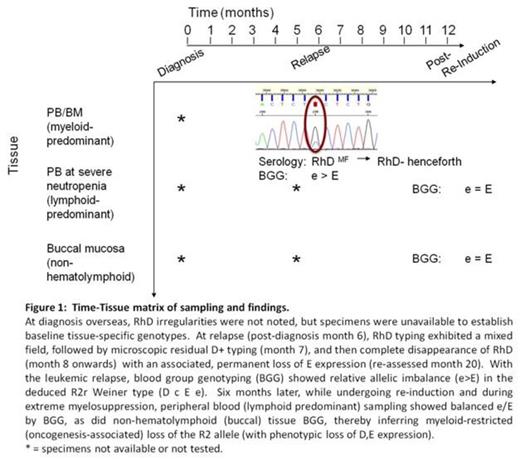
Six months after dignosis, eleven weeks after last red cell transfusion (pRBC), and 44 days after the last blood bank sample, his AML relapsed and he was then found to have mixed field reactions on RHD grouping, with two distinct populations of O+ and O- cells. Cytogenetic re-analysis of marrow did not demonstrate any abnormalities by G-banding.
Methods
Relapse versus post-re-induction bone marrow specimens with buccal mucosa serving as the non-hematopoeitic control were assessed by RH genotyping and chromosome 1p microsatellite mapping to deduce the basis for leukemia-associated loss of RHD expression.
Results
Genotyping analysis of peripheral blood demonstrated the RH genotype ccEe with D (R2r), although the ratio of e/E was out of the usual range for heterozygosity, with e predominant over E. Repeat testing with alternative sequencing primers ruled out unequal amplification of allele-specific polymorphisms at primer sites, while the absence of other out–of-range data for other antigens excluded chimerism. With the mixed field reactions indicating loss of the RHD, this suggested a specific deletion of an entire DcE (R2) allele at a clonal pre-erythroid level.
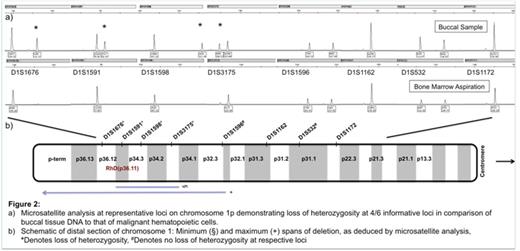
Microsatellite analysis on DNA extracted from non-myeloid tissue (buccal mucosa) compared to DNA extracted from bone marrow sample at relapse demonstrated loss of heterozygosity (LOH) at four of six informative loci, allowing for mapping of a putative chromosomal region of deletion (Figure 2).
Conclusions
LOH on chromosome 1 has been shown to be an important mechanism of RHD loss.[1] While such loss may be benign, the role of LOH in leukemogenesis has also been demonstrated,[2] as in this case. Alteration in RH expression may be either a surrogate for relapsed malignancy or the effect of an ongoing clonal evolutionary process.
[1] Kormoczi GF, Dauber EM, Haas OA, Legler TJ, Clausen FB, Fritsch G, Raderer M, Buchta C, Petzer AL, Schonitzer D, Mayr WR, Gassner C. Mosaicism due to myeloid lineage restricted loss of heterozygosity as cause of spontaneous Rh phenotype splitting. Blood 2007;110: 2148-57.
[2] Lahortiga I, Vazquez I, Belloni E, Roman JP, Gasparini P, Novo FJ, Zudaire I, Pelicci PG, Hernandez JM, Calasanz MJ, Odero MD. FISH analysis of hematological neoplasias with 1p36 rearrangements allows the definition of a cluster of 2.5 Mb included in the minimal region deleted in 1p36 deletion syndrome. Hum Genet 2005;116: 476-85
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal