Abstract
Intravenous Immunogloblulin (IVIG) is a plasma protein used in the treatment of various diseases. The cost of IVIG is approximately $50-$80 per gram. In 2005, Saskatchewan's usage of IVIG was above the national average. There are several published guidelines that outline recommended uses of IVIG. These are largely based on case studies and expert opinion. Despite these guidelines, IVIG is often used in multiple settings where there is no supporting evidence.
In 2013, we conducted a retrospective study on IVIG usage and found that the Saskatoon Health Region (SHR) adhered poorly to published guidelines. Following this, we aimed to determine IVIG usage in the Hematology and Neurology departments after implementing education sessions and order forms. We conducted six education sessions from December 2012 to January 2014 in the SHR. We also circulated IVIG order forms outlining appropriate indications for IVIG on the neurology and heme-oncology ward from January 1, 2014 to March 31, 2014. We then conducted a retrospective chart review of patients who received IVIG from January 1, 2014 to March 31, 2014 and compared the usage to patients who received IVIG in 2011 and 2012. Our primary endpoint was any improvement in the appropriate usage. Our secondary endpoint was adverse reactions seen in patients receiving IVIG. Statistical analysis was conducted with the use of two sample z-test for proportions.
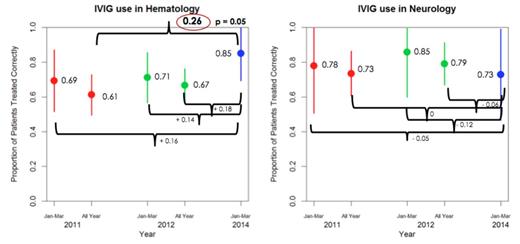
Our results (Figure 1) showed that for hematological conditions, the proportion of patients treated appropriately increased from 0.61 in 2011 to 0.85 in the first three months of 2014 (P value = 0.05). For neurological conditions, the proportion of patients treated appropriately declined from 0.85 in the first three months of 2012 to 0.72 in the first three months of 2014 (P value = 0.52). No adverse reactions were reported.
In conclusion, our results show that after our interventions, there was significant improvement in the appropriate use of IVIG for hematologicalconditions compared to 2011. However, there was a decrease in appropriate use of IVIG for neurological conditions. Further analysis and larger sample size is required to determine the overall significance of our interventions.
IVIG use in Hematology and Neurology after interventions implemented
IVIG use in Hematology and Neurology after interventions implemented
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal