Abstract
T-cell lymphoma (TCL) is the malignant proliferation of CD3+ lymphocytes and represents the main type of non-Hodgkin's lymphoma. TCL patients are generally resistant to conventional chemotherapy and have poor clinical outcome. Therefore, biomarker commonly expressed and closely related to tumor progression needs to be further investigated in TCL, helping to develop targeted therapeutic approaches and to eventually improve prognosis of the patients.
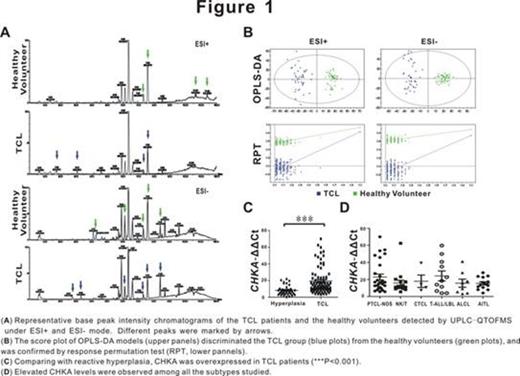
Growing evidences show that metabolic enzymes regulate key oncogenic signaling pathways and play an essential role on tumor progression. Here serum metabolomic analysis was performed in 45 patients with T-cell lymphoma (TCL) and 50 healthy volunteers, and the results showed that dysregulation of choline metabolism occurred in TCL. (Figure 1A-B) Further verified by real-time PCR, choline kinase-¦Á (CHKA), the main regulatory enzyme of choline metabolism, was overexpressed in patients with TCL, but without significant difference in the TCL subtypes. (Figure 1C-D)
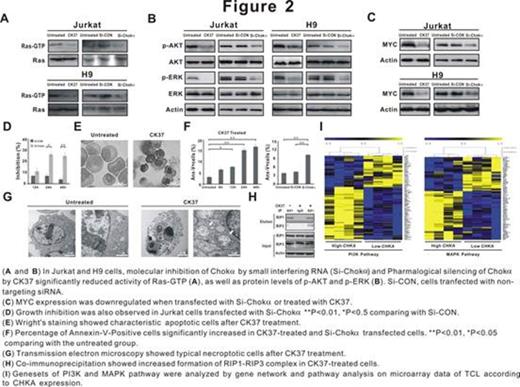
In T-lymphoma cells, pharmacological and molecular silencing of Chok¦Á significantly decreased Ras-GTP activity, AKT and ERK phosphorylation, MYC oncoprotein expression (Figure 2A-C), leading to induction of tumor cell apoptosis and necropotosis (Figure 2D-H). Clinically, gene expression profile analyzed in tissue samples of TCL patients showed that altered genes were enriched in PI3K and MAPK pathways according to CHKA expression of the TCL cases (Figure 2I).
In a T-lymphoma xenograft murine model, Chok¦Á inhibitor CK37 remarkably retarded tumor growth (Figure 3A), and SUV intensity of the CK37-treated tumors was significantly decreased on the figure of 18F-FDG small-animal PET/CT (Figure 3B). As in vitro study, comparing with the untreated group, p-AKT, p-ERK, MYC expression, and activity of Ras-GTP were significantly decreased, while in situ cell apoptosis/necropotosis increased in the tumor of the CK37 group. (Figure 3C-F)
To our knowledge, our report is the first time to analyze serum metabolomic profile in TCL patients, and provides direct evidence of aberrant choline metabolism in this disease. Importantly, dysregulation of choline metabolism was present in all histological types of TCL studied, consistent with acute myeloid leukemia that metabolomic status is independent on morphological classification or genetic feature. Therefore, manifested with a heterogeneous group of disease, TCL may share the similar profile of disturbed choline metabolism and this metabolic pathway can be exploited as a source of biomarkers and a potential therapeutic target in TCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal