Abstract
BACKGROUND Trials of ibrutinib and idelalisib for Chronic Lymphocytic Leukemia (CLL) targeting Btk and PI3-kinase respectively, illustrate the potential of targeting components of the B-cell receptor (BCR) signalling pathway. Emerging evidence suggests that subgroups of CLL patients develop resistance to these agents. In particular late relapses on ibrutinib appear to be associated with a high frequency of acquired mutations in Btk and PLCg2. Identification of novel combination therapies or agents that target multiple molecules in key signaling pathways represents a rational approach in the development of novel treatment strategies. Pim (provirus integration site for Moloney murine leukemia virus) family proteins are proto-oncogenic and involved in B-cell development and lymphoid malignancies. They are highly conserved serine/threonine kinases and are overexpressed in CLL. Given the clinical efficacy of idelalisib and results of preclinical studies of the PIM kinase SGI-1776 [Chen et al., 2009], we sought to investigate the potential of simultaneous inhibition of PIM and PI3-kinase for CLL therapy.
METHODS The effects of the novel inhibitor of PIM kinase, pPIMi, and the dual inhibitor of PIM/PI3-kinase, IBL-202 (both from Inflection Bioscience Ltd, Ireland), were investigated against panels of primary CLL patient samples and haematological lines. The in vitro efficacy of both agents was compared to that of SGI-1776 and idelalisib and studied under conditions involving CLL cell co-culture using CD40L-expressing fibroblasts that mimic the support offered by the CLL tumor microenvironment.
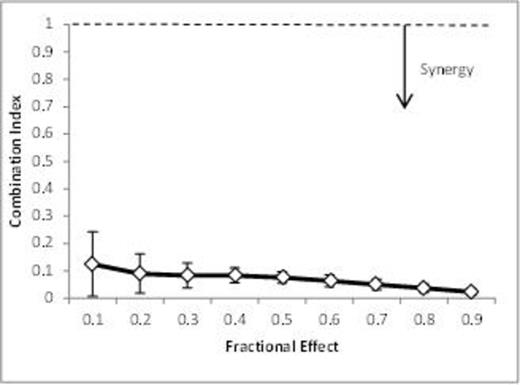
RESULTS Inhibition of PIM kinase by pPIMi was as effective as SGI-1776, inducing a dose dependent decrease in CLL-cell viability. The rationale for dual inhibition of PIM and PI3-kinase was investigated by combining pPIMi with idelalisib and by calculation of combination indices (CI), where CI values of < 1 are indicative of synergy. In primary CLL patient samples (Figure 1) and 3 B-cell lines (Mec1, Ramos and Raji) the combination proved highly synergistic, with CI values of 0.07 + /- 0.03 and 0.11 +/- 0.07 respectively, for fractional cell killing effects of between 0.1 and 0.9.
Consistent with our assessment of synergy between pPIMi and idelalisib, dual inhibition of PIM and PI3-kinase by IBL-202 was significantly more effective at inducing cell death than either pPIMi or idelalisib as single agents (Figure 2), evidenced by the significantly lower IC50 against CLL patient samples (7.47 µM, 43.12 µM, 1.23 µM for pPIMi, idelalisib and IBL-202 respectively).
The rationale for targeting PIM and PI3-kinase using IBL-202 was further highlighted by culturing CLL cells in the presence of a CD40L-expressing fibroblast monolayer, which represents a model of the tumor microenvironment. CD40L fibroblast co-culture significantly increased CLL-cell survival under control conditions but also conferred resistance to the PIM inhibitor pPIMi. In contrast CLL cells cultured under these conditions were sensitive to IBL-202 in a dose dependent manner.
CONCLUSION Inhibition of PIM and PI3-kinase is potently synergistic against CLL cells and is particularly effective under conditions that mimic the tumor microenvironment. Dual targeting of PIM and PI3-kinase by IBL-202 may represent a novel therapeutic strategy for CLL that minimizes the risk of acquired mutations associated with mono-molecular inhibition of BCR-signaling components.
Synergy between pPIMi and idelalisib in primary CLL patient samples supports dual inhibition of PIM and PI3-kinase by IBL-202.
Synergy between pPIMi and idelalisib in primary CLL patient samples supports dual inhibition of PIM and PI3-kinase by IBL-202.
Dose response analyses of pan PIM inhibitor (pPIMi), idelalisib (CAL-101) and PIM/PI3-kinase inhibitor (IBL-202) against primary CLL patient samples (n = 9).
Dose response analyses of pan PIM inhibitor (pPIMi), idelalisib (CAL-101) and PIM/PI3-kinase inhibitor (IBL-202) against primary CLL patient samples (n = 9).
O'Neill:Inflection Biosciences: Employment, Equity Ownership. O'Dwyer:Inflection Biosciences Ltd: Membership on an entity's Board of Directors or advisory committees. Mulligan:Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi Aventis: Research Funding; Janssen: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal