Abstract
Purpose
Essential thrombocythemia (ET) is rare in children. The clinical course and pathogenesis of childhood ET is far less clear. We aimed to analyze the clinical and molecular characteristics of children diagnosed with ET.
Patients and Methods
Sixty-three children diagnosed with ET (age < 15 years) were enrolled in the study. JAK2 V617F mutation was detected using quantitative real-time polymerase chain reaction. MPL and CALR mutations were assessed using Sanger sequencing. Fifty-five genes associated with myeloid malignancies were analyzed using targeted next-generation sequencing in 25 children.
Results
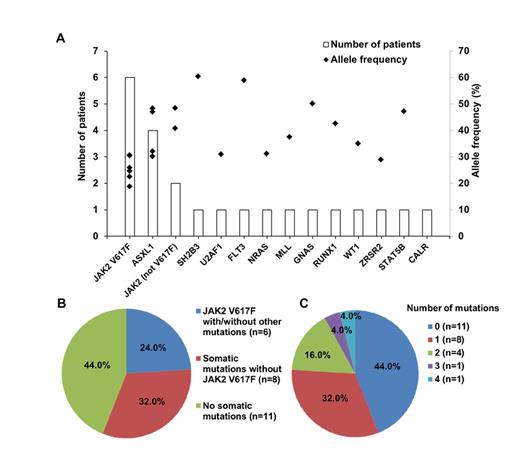
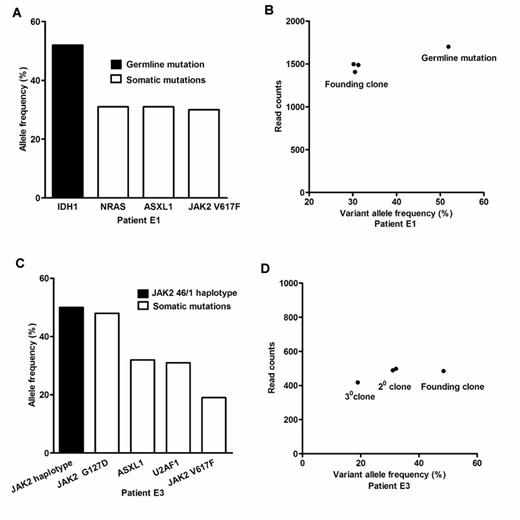
The median follow-up time for 63 patients was 48 months (range, 12 to 302 months). Three children (4.8%) had thrombosis, and 2 (3.2%) progressed to myelofibrosis. One child developed both abdominal vein thrombosis and myelofibrotic transformation. JAK2 V617F mutation was found in 14 children (14/63, 22.2%). CALR mutations (1/49, 2.0%) and MPL mutations (0/49) were rarely detected. By targeted sequencing, somatic mutations were found in 13 genes and in 56.0% (14/25) of the patients (Figure 1A and 1B). After JAK2 V617F, the most frequently observed mutations were found in ASXL1 (4/25, 16.0%). Besides, mutations in U2AF1, FLT3, NRAS, MLL, GNAS, RUNX1, WT1, and ZRSR2 were first reported in childhood ET. These newly identified mutations, which are rarely seen in adult ET, are linked to more malignant diseases such as acute leukemia and myelodysplastic syndrome. The key pathway involved was the JAK2 signaling pathway, followed by epigenetic regulators. In 14 children with somatic mutations, 42.9% (6/14) had two or more mutations (Figure 1C). Co-occurrence of mutations in the JAK2 signaling pathway and epigenetic regulators was found in 4 children (4/14, 28.6%). Clonality assessment revealed that some children only had founding clones, but some had both founding clone and subclones (Figure 2). Analysis of allele frequencies showed that the JAK2 V617F mutation and CALR mutations were not the initial abnormalities in some children.
Conclusion
Childhood ET has a distinct molecular profile from that of adult patients. CALR and MPL are rarely mutated in childhood ET; instead, some previously undocumented mutations are detected. Genetic composition in childhood ET may be more complex than that in adults. The JAK2 V617F mutation and CALR mutations were not the initial abnormalities in some children. Whole exome sequencing would help to reveal the initial molecular events and to offer molecular targets for therapeutic intervention.
Keywords
Childhood; Essential thrombocythemia; Molecular markers
Frequency and distribution of somatic mutations in children with essential thrombocythemia. (A) Number of patients and allele frequency of each mutated gene; (B) number of patients with JAK2 V617F mutation, with mutations except JAK2 V617F, and with no mutations; (C) number of patients with different number of somatic mutations.
Frequency and distribution of somatic mutations in children with essential thrombocythemia. (A) Number of patients and allele frequency of each mutated gene; (B) number of patients with JAK2 V617F mutation, with mutations except JAK2 V617F, and with no mutations; (C) number of patients with different number of somatic mutations.
Clonality assessment in two representative cases of essential thrombocythemia. (A) allele frequencies of mutations in Patient E1; (B) clusters of variants identify the founding clone in Patient E1; (C) allele frequencies of mutations in Patient E3; (D) clusters of variants identify the founding clone and subclones in Patient E3; in (B) and (D), the allele frequency is plotted versus the total number of sequencing reads covering the corresponding mutated nucleotide.
Clonality assessment in two representative cases of essential thrombocythemia. (A) allele frequencies of mutations in Patient E1; (B) clusters of variants identify the founding clone in Patient E1; (C) allele frequencies of mutations in Patient E3; (D) clusters of variants identify the founding clone and subclones in Patient E3; in (B) and (D), the allele frequency is plotted versus the total number of sequencing reads covering the corresponding mutated nucleotide.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal