Abstract

Background: The safety profile of each BCR-ABL1– targeted tyrosine kinase inhibitor (TKI) used to treat chronic myeloid leukemia (CML) is unique and should be considered when choosing therapy. Although rare, potentially severe adverse events have been reported in CML pts treated with TKIs, particularly pulmonary arterial hypertension with DAS (Montani, Circulation 2012), peripheral arterial occlusive disease with nilotinib (Kim, Leukemia 2013), and arterial and venous occlusive events with ponatinib (Cortes, N Engl J Med 2013). The incidence of CV ischemic events in DAS-treated pts from clinical trials was assessed. Standardized incidence rates (SIRs) were calculated to determine if the number of observed events was different than expected compared with reference populations.
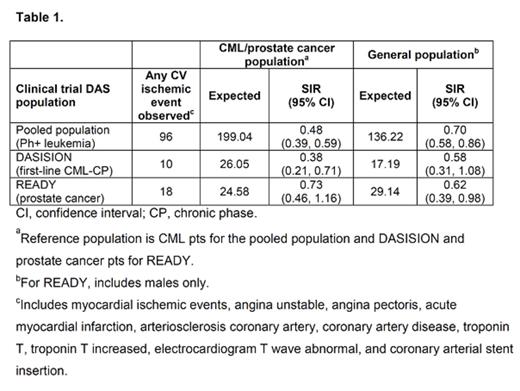
Methods: Incidence of CV ischemic events (Table 1) were assessed in a pooled population of DAS-treated pts with any phase CML or Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia from 11 first- and second-line clinical trials (N=2712; median age, 54 years [y]), newly diagnosed CML pts treated with DAS (n=258; median age, 46 y) or imatinib (IM; n=258; median age, 49 y) from the phase 3 DASISION trial only (CA180-056, NCT 00481247), and prostate cancer pts from the phase 3 READY trial treated with DAS or placebo in combination with docetaxel and prednisone (CA180-227, NCT 00744497; N=1518; about 66% of pts were >65 y of age). Reference populations for SIR analyses were a general population (total N=116,000,000; males N=56,000,000), CML pts (N=16,000), and prostate cancer pts (N=530,000) derived from Truven's MarketScan Commercial Claims and Medicare Supplemental database, 2008–2013, narrowed to mimic clinical trial eligibility. SIRs were calculated by dividing the observed number of events in DAS-treated pts by the expected number of events, based on the DAS exposure and reference population event rates.
Results: Within the pooled population, 96 pts (4%) had CV ischemic events. In DASISION, 10 DAS- and 4 IM-treated pts had any grade CV ischemic events. In READY, 18 pts in the DAS arm and 9 pts in the placebo arm had any grade CV ischemic events. The majority of pts with a CV event had a history and/or risk factors for atherosclerosis (77/96 [80%] in the pooled population; 8/10 with DAS and 3/4 with IM in DASISION). Time-to-event analysis revealed that, in the pooled population, CV ischemic events occurred in 69/96 pts (72%) within 1 y, 11 pts (11%) in 1–2 y, and 16 pts (17%) in 3–7 y. Over 70% tolerated continued DAS therapy without a recurrent CV event. In DASISION, CV events occurred in 7/10 pts within 1 y of DAS initiation, 2 pts in 1–3 y, and 1 pt after 5 y. Based on SIRs, the observed number of CV events in DAS-treated pts was not higher than expected, given the rates of reference populations (Table 1). SIR results should be interpreted with caution due to the limitations of the indirect comparison between clinical data and claims data (eg, coding differences and surveillance bias).
Conclusion: CV ischemic events were reported in 4% of DAS- and 2% of IM-treated pts in DASISION, 4% of DAS-treated pts in the pooled population, and 2% of DAS- and 1% of placebo-treated pts in READY. In all populations, among pts who experienced an event, the majority had a history of arterial ischemic events and/or risk factors for atherosclerosis, and most events occurred early. SIRs suggest that the total number of CV ischemic events among DAS-treated pts was not higher than expected, and in contrast to what has been observed with other TKIs, largely restricted to 1 y after initiating therapy.
Saglio:Pfizer: Consultancy, Fees for occasional speeches, Fees for occasional speeches Other; Novartis: Consultancy, Fees for occasional speeches, Fees for occasional speeches Other; ARIAD: Consultancy, Fees for occasional speeches, Fees for occasional speeches Other; BMS: Consultancy, Fees for occasional speeches Other. Off Label Use: Dasatinib is approved for first line use in adults with chronic phase Ph+ CML, and in adult AP- or BP-CML, and Ph+ ALL patients who are resistant or intolerant to prior therapy. le Coutre:ARIAD: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Honoraria. Cortes:Teva: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Ariad: Consultancy, Research Funding. Mayer:Novartis: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Mahon:ARIAD: Consultancy, Research Funding; Pfizer : Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Kroog:Bristol-Myers Squibb: Employment. Gooden:Bristol Myers Squibb: Employment. Subar:Bristol Myers Squibb: Employment. Preston:Bristol-Myers Squibb: Employment. Shah:ARIAD: Research Funding; Bristol-Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal