Abstract
Background. Peripheral T-cell lymphomas (PTCLs) originate from post-thymic T cells but compared to B-cell lymphomas the exact cell of origin is usually unknown except for angioimmunoblastic T-cell lymphoma arising from a follicular helper T-cell. Furthermore, no recurrent cytogenetic or molecular abnormalities are identified in PTCLs. Recently, recurrent impairment of the p53 pathway has been pointed out in PTCLs. However, p53 knockout (KO) mice are known to develop immature thymic T-cell lymphomas and solid tumors but surprisingly PTCLs have not been reported for more than 20 years in those mice.
NKT cells are a T-cell subset responsive to glycolipids presented by CD1d, a major histocompatibility complex (MHC) class I-like antigen-presenting molecule, in contrast to conventional T cells, which recognize peptide antigens. Two types of NKT cells have been described so far: type I or invariant NKT cells (iNKT) that express a Valpha14-Jalpha18 (in mice) or Valpha24-Jalpha18 (in humans) constant chain and type II NKT cells that express a variable TCR but are CD1d-dependent as well. Most type II NKT cells are of alpha/beta phenotype but CD1d-restricted gamma/delta T cells have also been described in mice and humans.
Methods. The development of PTCLs in p53 KO mice (B6.129S2-Trp53tm1Tyj/J) was studied. Identification of PTCLs was made by immunohistochemistry and flow cytometry analysis. Gene expression profile analysis (GeneChip Mouse Genome 430 2.0 array, Affymetrix) was performed to characterize lymphomas developed in the mouse model. Transfer experiments were done by intravenously retro-orbital injection into syngeneic, immunocompetent C57Bl/6J WT animals or immunocompromised CD3ε-/- mice. Therapeutic trials in mice were performed with the use of blocking anti-CD1d monoclonal antibodies (mAb) (clone HB323; BioXcell).
Results. We found that p53 KO mice developed well-known and characterized thymic T-cell lymphomas and solid tumors as previously described. However, about 20% of p53 KO mice spontaneously developed a previously unrecognized entity of PTCLs originating from CD1d-restricted iNKT cells (ie type I NKT cells) referred to as NKTLs for NKT lymphomas thereafter. Both alpha-galactosylceramide-CD1d tetramer staining and unique Valpha14-Jalpha18 TCR rearrangement confirmed the iNKT nature of these lymphomas.
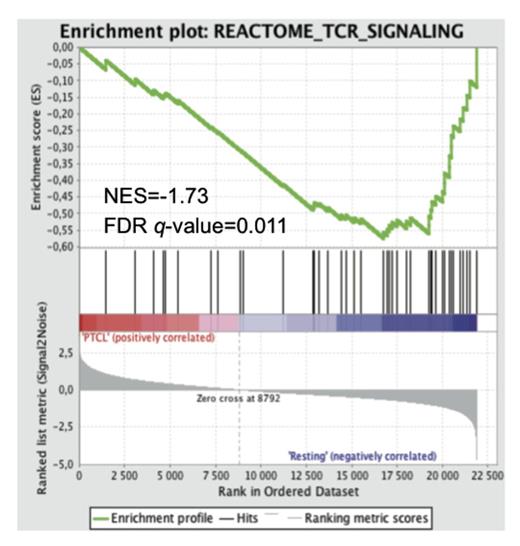
Chronic injection of Streptococcus pneumoniae (Spn), reported to express glycolipid antigens activating NKT cells, significantly increased the incidence of these NKTLs compared to a control group of p53 KO mice injected with PBS (P=0.03). Gene expression profile analysis indicated a significant down-regulation of genes in the TCR signaling pathway of NKTLs (false discovery rate q-value=0.01 by gene set enrichment analysis) suggesting an underlying antigenic chronic stimulation as previously reported in chronically activated T cells (Figure 1). Moreover, NKTLs were characterized by upregulation of PD-1 and loss of NK1.1 expression compared to resting NKT cells (P<0.01 for both), which are features of activated and anergic iNKT cells. Altogether, those data indicate that NKTLs in mice could arise from chronically activated iNKT cells by endogenous or exogenous glycolipids. Furthermore, in vivo TCR/CD1d interactions were required for NKTLs survival after transfer in recipient mice and the use of blocking anti-CD1d mAb significantly prolonged mice overall survival (logrank P<0.001, Figure 2).
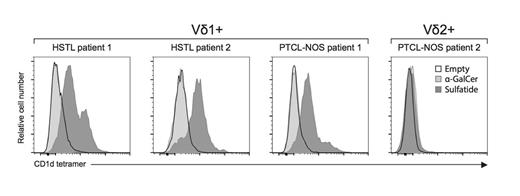
We did not identify human PTCLs arising from type I iNKT cells by using alphaGalCer-CD1d tetramer staining. However, using sulfatide-loaded CD1d tetramers (ie another type of glycolipid-CD1d tetramer identifying type II NKT cells), we identified CD1d-restricted human PTCLs among gamma/delta hepatosplenic T-cell lymphomas (HSTLs) and PTCL-not otherwise specified (PTCL-NOS) expressing the Vd1 TCR but not the Vd2 TCR (Figure 3).
Conclusion. This demonstrates for the first time the existence of human PTCLs arising from gamma/delta CD1d-restricted type II NKT cells. These results refine the classification of PTCLs in humans by identifying a new cell of origin and pave the way for the development of blocking anti-CD1d antibodies for therapeutic purposes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal