Abstract
INTRODUCTION
Siltuximab (SylvantTM), a monoclonal antibody against interleukin-6, was recently approved in both US and EU for treatment of adult patients with Multicentric Castleman's Disease (MCD) who are human immunodeficiency virus (HIV) negative and human herpesvirus-8 (HHV-8) negative. Information on MCD symptoms, functioning and well-being was gathered in a pivotal global registration study (MCD2001, Van Rhee 2014) with a general patient reported outcome (PRO) questionnaire: the Short Form-36 (SF-36, acute version 2), and other PRO measures.
OBJECTIVES
Evaluate the longitudinal impact of siltuximab on symptoms, functioning and well-being as measured by SF-36 when compared to placebo.
METHODS
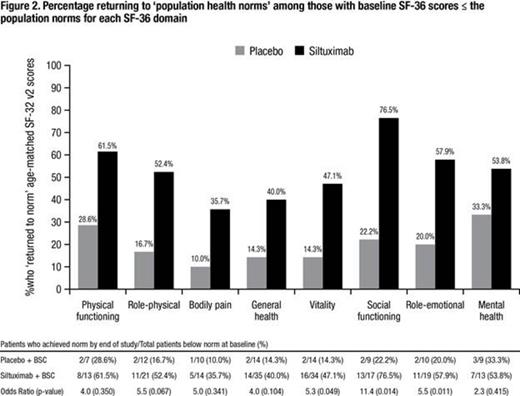
MCD2001 was a randomized (2:1), double-blind, placebo-controlled, multicenter study to determine the safety and efficacy of siltuximab in patients with symptomatic MCD. Patients received siltuximab 11 mg/kg or placebo by intravenous infusion every 3 weeks in combination with best supportive care (BSC). Subjects (N = 79) completed the SF-36 at baseline and every three weeks throughout the treatment period; 1998 scoring weights were applied. Patients, whose baseline SF-36 domain scores were below the general population norm for their age group (n = 46) were included in an analysis to compare by treatment arm the proportion of patients achieving an SF-36 domain score equal to, or greater than, the population norm at final visit. This analysis, hereafter referred to as a return to general population health norms (RTN), was conducted separately for each SF-36 domain. It is regarded as clinically-relevant improvement (CRI) since disease control is the current treatment goal. Referent norms were based on the US general population SF-36 cohort from the Medical Outcomes Study (Ware et al 1998). An intent-to-treat analysis was applied where missing data were not imputed; data were censored at treatment discontinuation, including crossover. Odds ratio was calculated between the two treatment arms and statistically compared with the Fisher’s Exact test.
RESULTS
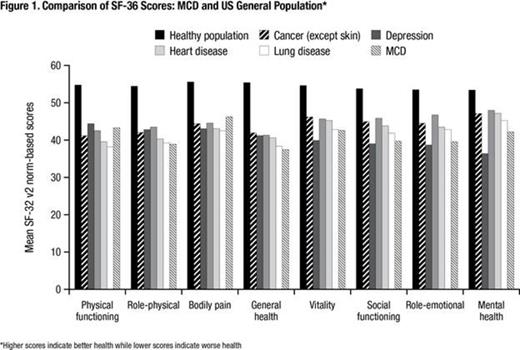
At baseline the population demonstrated inferior health compared to general population across all SF-36 domains (Figure 1). The odds of siltuximab-treated patients for reaching RTN vs. placebo patients in the Vitality, Role-emotional and Social functioning domains are significant, with odds ratios of 5.3 (p=0.049), 5.5 (p=0.011) and 11.4 (p=0.014), respectively. A positive health trend was observed for patients who received siltuximab compared with placebo across all other domains, particularly Role-physical (5.5, p=0.067; Figure 2). Sensitivity analysis using a literature-based CRI supported the robustness of the results.
CONCLUSION
Overall MCD patients reported notably inferior health compared to the general population at baseline; siltuximab significantly improved the odds for RTN versus placebo in the Role-emotional, Vitality and Social function domains, as well as demonstrated a trend to improve health across all other SF-36 domains among MCD patients who were below the norms at baseline. A clinically-relevant PRO benefit for physical and mental health was observed with siltuximab therapy, as measured with the SF-36, which supports the “disease control” treatment goal.
References
Van Rhee et al. Siltuximab for multicentric Castleman’s disease:a randomised, double-blind, placebo-controlled trial, Lancet Oncol, 2014, 15(9):966-74
Teschendorf B et al. Development and Content Validation of a Multicentric Castleman’s Disease Symptom Scale; Value in Health, 2010, 13(7): A470
Ware et al. SF-36v2 health survey: manual and interpretation guide. 1998
van Rhee:Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Casper:Janssen: Consultancy, Research Funding. Robinson:Johnson & Johnson: Employment, Stocks Other. He:Janssen: Employment, Equity Ownership. Vermeulen:Janssen Biologics: Employment, Equity Ownership, Honoraria. van de Velde:Janssen Research & Development: Employment, Equity Ownership. Wong:Johnson & Johnson: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Research Funding; Biogen-Idec: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal