Abstract
Bendamustine is an alkylator containing a benzimidazole ring; it only has partial cross-resistance with the other alkylating agents. Several studies have shown that bendamustine is effective as a single agent or as combined treatment with rituximab for low-grade B-cell lymphoma. Although bendamustine has a favorable safety profile, the occurrence of myelosuppression and lymphocytopenia is relatively frequent. In lymphocytopenia, CD4-positive (CD4+) T-cell recovery is specifically impaired. If this occurs over a prolonged period of time, patients are at risk of opportunistic infections, such as cytomegalovirus (CMV) reactivation. This is a risk for bendamustine-treated patients, although the actual frequency of CMV reactivation in these patients is not clear.
In this study, we prospectively evaluated the occurrence of CMV reactivation in relapsed or refractory low-grade B-cell lymphoma patients who were treated with bendamustine alone or in combination with rituximab. We simultaneously investigated the immune state of the patients. We analyzed CMV pp65 antigen in leukocytes, immunoglobulin (Ig) G, IgM, and IgA levels, and the CD4+:CD8+ lymphocyte ratio prior to treatment and after the third and sixth courses of treatment.
There were 29 patients enrolled in the study. The median age was 68 years old (range, 28–83 years). Patients were treated a median of 2 times prior to bendamustine treatment; these treatments included chemotherapy and radiotherapy. There were 15 patients (51%) who completed 6 courses of chemotherapy.
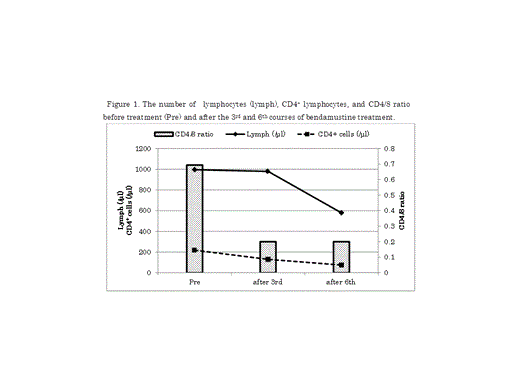
The immune status of the patients is shown in Figure 1. The median CD4+ lymphocyte count prior to treatment was 218/µL, which decreased to 75/µL by the end of treatment. The median of IgG, IgM, and IgA concentrations before treatment were 909, 52, and 147 mg/dL, respectively; by the end of treatment, the IgG, IgM, IgA concentrations had decreased to 832, 30, and 110 mg/dL, respectively. The extent of CD4+ T-cell depression during treatment appeared to be more severe than the Ig depression.
CMV pp65 antigen was detected in 3 patients after the third course of treatment, and in 2 patients after the sixth course of treatment. None of the patients had any symptoms and CMV pp65 antigen count returned to negative without any treatment. The median CD4+ lymphocytes count in patients with or without CMV reactivation is shown in Table 1. There is a trend that the depression of CD4+ cells after the 6th course of bendamustine increases the risk of CMV reactivation.
This is the first study to prospectively analyze CMV reactivation in low-grade B-cell lymphoma patients treated with bendamustine. In this study, we found that CMV reactivation by bendamustine occurred in approximately 15% of patients. On the other hand, the risk of symptomatic CMV disease, which would require medical intervention, does not appear to be high in bendamustine-treated patients.
Most patients with relapsed or refractory low-grade B-cell lymphoma who are administered bendamustine therapy are in an immunosuppressed condition at the beginning of treatment. This is due to heavy chemotherapy pretreatment, which is often performed using rituximab. In addition to this, the decrease in CD4+ lymphocytes observed during bendamustine treatment, is likely to increase the risk of opportunistic infections, such as CMV reactivation. In future studies, we aim to investigate the risk factors for symptomatic cytomegalovirus infection in patients treated with bendamustine, especially in combination with rituximab.
We hope that our study, which evaluates the risk of opportunistic infections in bendamustine treated patients, will be beneficial in determining the optimal timing for a preemptive approach for CMV infection. This will be helpful in establishing safe bendamustine treatment regimens in relapsed or refractory low-grade B-cell lymphoma patients.
The median number of lymphocytes, CD4+ lymphocytes and CD4/8 ratio in patients with or without cytomegalovirus reactivation
| . | Parameter . | All patients . | CMV reactivation . | p value . | |
|---|---|---|---|---|---|
| + . | - . | ||||
| Before treatment (n=24) | Lymph (/µl) | 996 | 1518 (n=5) | 987 (n=19) | 0.24 |
| CD4+ cells (/µl) | 218 | 261 | 218 | 0.96 | |
| CD4/8 ratio | 0.69 | 0.60 | 0.69 | 0.54 | |
| After the 3rd course (n=23) | Lymph (/µl) | 980 | 1330 (n=5) | 944 (n=18) | 0.39 |
| CD4+ cells (/µl) | 130 | 118 | 130 | 0.52 | |
| CD4/8 ratio | 0.20 | 0.12 | 0.20 | 0.16 | |
| After the 6th course (n=15) | Lymph (/µl) | 628 | 579 (n=5) | 902 (n=10) | 0.27 |
| CD4+ cells (/µl) | 75 | 36 | 118 | 0.035 * | |
| CD4/8 ratio | 0.20 | 0.17 | 0.21 | 0.35 | |
| . | Parameter . | All patients . | CMV reactivation . | p value . | |
|---|---|---|---|---|---|
| + . | - . | ||||
| Before treatment (n=24) | Lymph (/µl) | 996 | 1518 (n=5) | 987 (n=19) | 0.24 |
| CD4+ cells (/µl) | 218 | 261 | 218 | 0.96 | |
| CD4/8 ratio | 0.69 | 0.60 | 0.69 | 0.54 | |
| After the 3rd course (n=23) | Lymph (/µl) | 980 | 1330 (n=5) | 944 (n=18) | 0.39 |
| CD4+ cells (/µl) | 130 | 118 | 130 | 0.52 | |
| CD4/8 ratio | 0.20 | 0.12 | 0.20 | 0.16 | |
| After the 6th course (n=15) | Lymph (/µl) | 628 | 579 (n=5) | 902 (n=10) | 0.27 |
| CD4+ cells (/µl) | 75 | 36 | 118 | 0.035 * | |
| CD4/8 ratio | 0.20 | 0.17 | 0.21 | 0.35 | |
*; significant
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal