Abstract
Background:
The phase 3, randomized LYM-3002 study compared VR-CAP (n=243) vs R-CHOP (n=244) as frontline therapy in newly diagnosed MCL pts who were ineligible or not considered for bone marrow transplantation (NCT00722137). In the primary analysis after a median follow-up of 40 mos, progression-free survival (PFS) as assessed by independent radiology review committee (IRC) (median 24.7 vs 14.4 mos; hazard ratio [HR] 0.63; p<0.001) or by investigator (median 30.7 vs 16.1 mos; HR 0.51; p<0.001) was significantly prolonged with VR-CAP vs R-CHOP. A trend for improved OS (a pre-specified secondary endpoint) was also seen with VR-CAP vs R-CHOP (median not reached vs 56.3 mos; HR 0.80; p=0.173) and 4-yr OS rates were 64.4% vs 53.9% (Cavalli et al. ASCO 2014, Abs 8500; Robak et al. EHA 2014, Abs S1345). An association between higher Btz cumulative dose and improved OS has been observed in newly diagnosed multiple myeloma pts (Mateos et al. ASH 2013, Abs 1968). This post-hoc analysis of LYM-3002 evaluated the impact of Btz dose intensity on OS in newly diagnosed MCL pts receiving VR-CAP.
Methods:
In the VR-CAP arm, pts with measurable stage II–IV MCL and an ECOG performance status (ECOG PS) of 0–2 received 6–8 × 21-d cycles of Btz 1.3 mg/m2 IV on d 1, 4, 8, and 11, plus rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, all IV on d 1, and prednisone 100 mg/m2 PO on d 1–5. Btz dose adjustments for toxicities were permitted using established dose modification guidelines per the drug prescribing information. OS was estimated by Kaplan-Meier methodology. For this analysis, Btz dose intensity (total Btz dose received per cycle) during cycles 1–6 was calculated. The median value was selected as a cut-off for dichotomization of pts to be included in lower or higher (<4.6 vs ≥4.6 mg/m2/cycle) dose intensity groups. OS was then compared between groups, among pts who had received ≥6 cycles of Btz (n=181) in a landmark analysis from the end of cycle 6. The impact of potential confounding factors (baseline factors and on-treatment changes in response status, ECOG PS, and nadir platelet/neutrophil counts pre-landmark) was evaluated in multivariate analysis using Cox proportional hazards modeling.
Results:
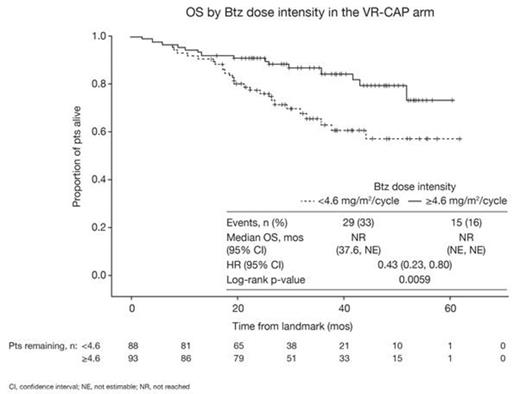
From the landmark, OS was found to be significantly longer in the higher (n=93) vs lower (n=88) Btz dose intensity group in univariate analysis (HR 0.43 [0.23, 0.80]; p=0.0059; Figure). 4-yr OS rates were 79.5% and 57.1%. In multivariate analysis adjusted for potential confounding baseline patient/disease characteristics that may have been imbalanced between the dose groups (age [≤65 vs >65 yrs], sex, race [white vs non-white], ECOG PS [≤1 vs >1], disease stage [II/III vs IV], extranodal involvement [≤1 vs >1], LDH level, white blood cell count, bone marrow involvement, albumin level, platelet count), Btz dose intensity remained the most significant predictor of OS (HR 0.40 [0.20, 0.79]; p=0.009). After selection for the most significant covariates (Btz dose intensity, ECOG PS, LDH level), dose intensity remained the strongest predictor of OS of all variables considered in the model (HR 0.38 [0.20, 0.71]; p=0.003). Dose intensities of other drugs in the VR-CAP regimen showed no association with OS due to infrequent dose modification. After adjusting for achievement of complete response (CR)/unconfirmed CR (CR/CRu) by investigator assessment (per International Lymphoma Workshop Criteria) by the end of cycle 6 (yes [n=28] vs no [n=151]), which showed no impact on OS (HR 0.36 [0.11, 1.17]; p=0.09), the impact of Btz dose intensity on OS remained highly significant (HR 0.43 [0.23, 0.80]; p=0.007). Similar findings were observed after adjusting for achievement of CR/CRu by IRC. Adjusting for change from baseline to landmark in ECOG PS (worsening [n=26] vs non-worsening [n=153]; HR 0.88 [0.40, 1.95]; p=0.76), and worst on-treatment platelet count (≥25 [n=120] vs <25 [n=59] x109/L; HR 0.72 [0.38, 1.37]; p=0.31) or neutrophil count (≥0.75 [n=38] vs <0.75 [n=141] x109/L; HR 1.47 [0.59, 3.68]; p=0.41), showed no significant impact on OS of these variables, but demonstrated continued prognostic significance of Btz dose intensity.
Conclusions:
Higher Btz dose intensity was the strongest predictor of OS in newly diagnosed MCL pts receiving frontline VR-CAP. The association was independent of baseline characteristics, dose intensity of co-administered drugs, or on-treatment changes in response status or hematologic parameters.
Robak:Janssen Research & Development: Consultancy, Research Funding. Off Label Use: The proteasome inhibitor bortezomib is approved in the US for the treatment of multiple myeloma and for the treatment of patients with mantle cell lymphoma who have received at least one prior therapy. In the present study, bortezomib is being investigated in combination with immunochemotherapy for previously untreated patients with mantle cell lymphoma, an indication for which it is currently not approved.. Siritanaratkul:Novartis: Research Funding; Roche: Research Funding; Janssen: Research Funding. Mayer:Janssen Research & Development: Research Funding; Roche: Research Funding; GlaxoSmithKline: Research Funding; Celgene: Research Funding. Pei:Janssen: Employment. Rooney:Johnson & Johnson: Equity Ownership; Janssen: Employment. van de Velde:Johnson & Johnson: Equity Ownership; Janssen: Employment. Cavalli:Roche: Research Funding; Takeda: Consultancy; Pfizer: Research Funding; Mundipharma: Research Funding; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal