Abstract
PNH is a rare acquired clonal disorder of the hematopoietic stem cell, characterized by a somatic mutation that inactivates the X-linked PIGA gene: this in turn results in deficiency on the cell surface of all proteins anchored by the glycosylphosphatidylinositol (GPI) molecule. Two of these proteins,CD55 andCD59, are complement regulators and their deficiency is responsible for the susceptibility of red cells (RBCs) from the mutant clone to lysis by activated complement. Since oxidative damage is another well-known mechanism of hemolysis (as in G6PD deficient red cells), we have investigated whether this plays a role also in PNH.
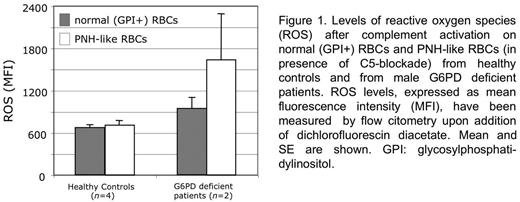
To this end, we have carried out experiments on RBCs from healthy donors and on PNH-like RBCs (obtained in vitro from the same donors through the use of anti-CD55 and anti-CD59 blocking moAb). After exposure to AB0-compatible serum (in which thecomplement alternative pathway was activated by mild acidification) all PNH-like (but not normal) RBCs were lysed. In parallel experiments in which complement was blocked by eculizumab (ECU) - a moAb that binds to the complement component C5 and controls intravascular hemolysis in PNH patients - we measured the levels of reactive oxygen species (ROS) by the dichlorofluorescin diacetate assay. We found no significant difference of ROS levels between normal RBCs and PNH-like RBCs. We next tested in a similar way G6PD-deficient RBCs, because these are known to be exquisitely sensitive to oxidative damage. We found that ROS levels were significantly higher in the G6PD deficient RBCs that have been made PNH-like (Fig. 1). Thus, complement activation on the surface of PNH-like RBCs results in the production of ROS that can be demonstrated when C5-blockade prevents complement-mediated lysis of RBCs.
The notion that G6PD deficiency can interact with PNH was strongly corroborated by the clinical observation of a 40yo woman from Sardinia (Italy) with a 2 years history of pancytopenia, who then developed florid hemolytic PNH: she had anemia with normal granulocyte and platelet counts, dark urine, high reticulocytosis, LDH up to 5x upper normal level, 95% GPI-negative granulocytes. When the patient was started on ECU. LDH levels promptly returned to normal, PNH RBCs rose from 20% (before ECU) to 42%, but reticulocyte count (~250x109/L) and blood transfusion requirement remained high (10 units in the last year). 39% of the GPI-negative RBCs had bound C3 fragments The peripheral blood smear revealed marked macro-anisocytosis, poikilocytosis, spherocytes, and hemighosts: a picture consistent with oxidative damage as seen in G6PD deficient patients during a hemolytic attack. The RBC G6PD activity was about one-half of normal (5 IU/g Hb), and DNA analysis revealed heterozygosity for the G6PD Mediterranean (Med) mutation. By mRNA sequence analysis we found that the GPI-negative clone expressed only the G6PD Med allele, suggesting that the PIG-A mutation took place in a stem cell in which the normal G6PD gene was on the inactive X-chromosome (G6PD, like PIG-A, is on the X chromosome); therefore, all the patients' PNH RBCs were also all G6PD deficient.
We have previously shown that the clinical expression of PNH can be influenced by inherited factors: specifically, a polymorphism of the complement receptor 1 (CR1) gene correlates with the blood transfusion requirement of patients on ECU (Rondelli et al, Haematologica 2014). However, the patient here reported was homozygous for the more favorable allele ofCR1. Instead, in keeping with our experimental data, the poor response to ECU seen in this patient results probably from a unique interaction, within the same population of RBCs, between the acquired PNH abnormality and her inherited G6PD deficiency
This type of interaction is novel and it seems to have pharmacogenetic implications. Indeed, on its own G6PD deficiency affects mildly the clinical expression of PNH, because complement activation causes RBC lysis regardless; however, paradoxically, when the lysis of PNH RBCs is prevented by C5 blockade, complement activation results in oxidative damage, with which PNH G6PD deficient RBCs are unable to cope.
Except for one case previously reported by Oni et al (Blood 1970), this is the first detailed study of PNH associated with G6PD deficiency. Since in some parts of the world the frequency of G6PD deficiency can be as high as 30% or more, we expect that more cases of this association will be discovered in the future.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal