Abstract
Despite increasing use of targeted oral anticoagulants, vitamin K antagonists remain the mainstay of thromboembolism intervention. Vitamin K antagonists exert their anticoagulant effect by preventing formation of gamma-carboxylation of vitamin K dependent clotting factors. This effectively inhibits Gla domain-mediated protein conformational changes thereby altering protein-membrane binding required for robust thrombin generation and formation of a stable clot.
Disadvantages of vitamin K antagonists include a narrow therapeutic window, variable pharmacokinetics and annual bleeding rates of up to 15%. While available reversal strategies exist, they are limited by a) duration to achieve coagulopathy reversal precluding use of vitamin K in emergencies b) logistical barriers of elapsed time to administer fresh frozen plasma (FFP) and/or patient inability to tolerate volume required for reversal, and c) in the case of prothrombin complex concentrates, limited availability and potential thrombogenicity. No reversal strategy achieves rapid onset to efficacy and short duration of anticoagulation reversal. Rapid restoration of hemostasis with expeditious return to an anticoagulated state may prove beneficial in medically complex patients with conflicting hemostatic requirements, eliminating the need for bridging therapy and improved perioperative management.
We previously developed "zymogen-like" factor Xa (FXa) molecules with impaired active site maturation enabling a greater half-life (30 minutes) than wild type FXa (1-2 minutes) while maintaining full procoagulant function when assembled in the prothrombinase complex (Blood 2011, 117:290-8; Nat. Biotech 2011, 29:1028-33). We evaluated if one variant, FXaI16L, may reverse warfarin anticoagulation.
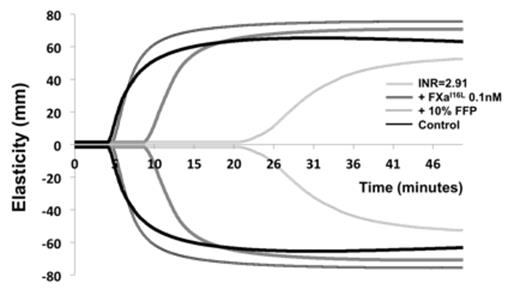
We analyzed whole blood specimens from 12 subjects on warfarin (median INR 2.61, range 2.35-3.89) by rotational thromboelastometry (ROTEM) initiated with 0.2 pM tissue factor (TF) in the presence of varying concentrations of FXaI16L. FXaI16L dose dependently corrected whole blood ROTEM parameters. Subject clot times with 1 nM FXaI16L did not significantly differ from controls. Analysis of samples with the addition of 10% volume FFP (intended to simulate administration of 3 FFP units in a 60 kg adult) improved clot time but was still significantly prolonged compared to both control and analysis with 1 nM FXaI16L (Figure 1). Our findings show that FXaI16L is able to correct ex vivo hemostatic abnormalities observed by ROTEM in warfarin whole blood and appears superior to FFP.
By effectively bypassing the coagulation cascade to assemble the prothrombinase complex, the ability of FXaI16L to restore ex vivo hemostasis in warfarin anticoagulated whole blood suggests un- or under-carboxylated prothrombin may not prohibit robust thrombin production. To explore this further, thrombin generation assays (TGA) were used to evaluate varying INR plasmas (INR 7.1, 4.1, 2.9) initiated with 0.5 pM TF, 4 μM phospholipid vesicles (PCPS) with or without increasing concentrations of FXaI16L. Minimal thrombin generation was observed in warfarin plasma in the absence of FXaI16L. Irrespective of plasma INR, 2 nM FXaI16L restored thrombin generation to approximate thrombin generated in normal human plasma under the same conditions without FXaI16L. To further determine if uncarboxylated (des-Gla) prothrombin can be activated in plasma, we reconstituted prothrombin deficient plasma with either des-Gla or carboxylated prothrombin. The addition of 2 nM FXaI16L to des-Gla prothrombin plasma generated 1/3 of thrombin observed in carboxylated prothrombin plasma. Our results are consistent with a modest decrease in the rate of thrombin generation from des-Gla prothrombin previously observed (J Biol Chem 2013, 288:27789-800).
These findings demonstrate that despite under-carboxylated prothrombin in warfarin plasma and whole blood, FXaI16L is able to effectively contribute to robust thrombin generation. This work suggests FXaI16L may serve as a quick onset, short acting warfarin reversal agent. A Phase I clinical trial is currently evaluating the safety of FXaI16L (ClinicalTrials.gov; NCT01897142). Additionally, our findings challenge the longstanding notion that fully carboxylated prothrombin is required for robust thrombin generation.
FXaI16L corrects ROTEM findings in warfarin whole blood and appears superior to FFP
FXaI16L corrects ROTEM findings in warfarin whole blood and appears superior to FFP
Camire:Pfizer: Consultancy, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal