Abstract
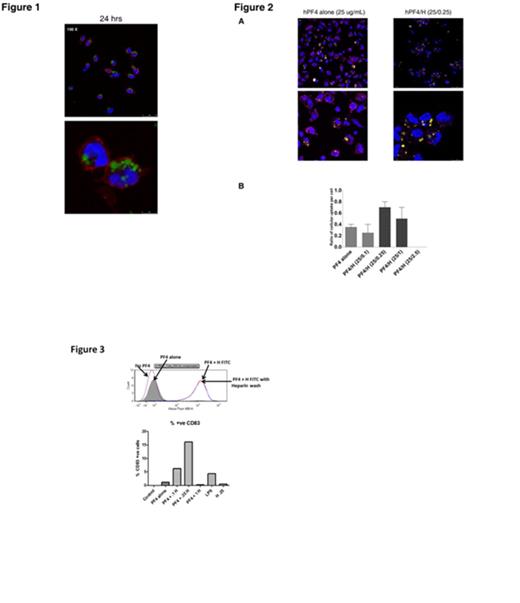
Platelet Factor 4 (PF4), a strongly positive-charged small protein, and the negatively-charged polymer heparin (Hep) form ultra-large complexes (ULCs) that have several unusual features including their remarkable stability and their ability to elicit a robust antibody response in vivo (Rauova L; Blood 2005 and Suvarna S, Blood 2005). Recently, we and others have shown that similar complexes of another positively charged protein, protamine sulfate with heparin can also lead to a clinically relevant immune response. To better understand the cellular basis for PF4/Hep antibody formation, we investigated mechanisms of cellular interactions and uptake of PF4 and PF4/Hep ULCs. For these studies, we examined cellular uptake of unlabeled or labeled PF4, heparin, or PF4/Hep using monocytes (PBMCs), dendritic cells (DCs) and/or neutrophils derived from peripheral blood. For these studies, cells were incubated with varying concentrations of unlabeled or fluorescently-labeled antigen (PF4, Hep or PF4/Hep ULCs). In cellular studies using unlabeled antigen, uptake was detected by fluorescently-labeled KKO, a monoclonal antibody to PF4/Hep complexes. Cellular uptake was visualized by confocal microscopy or flow cytometry. In initial studies, we defined the time course of uptake. As shown in Figure 1, we demonstrate that PF4/Hep-FITC ULCs are taken up by PBMCs in a time-dependent manner, with maximal uptake occurring between 12-24 hours (Figure 1, only 24 hour time point shown). This uptake is independent of the fluorescent label, as labeled or unlabeled intracellular PF4/Hep ULCs were readily visualized by KKO-AF647. To examine the effect of Hep on PF4 uptake, PBMCs were incubated with unlabeled PF4 alone or in the presence of increasing concentrations of Hep-FITC (0.1-2.5 U/mL). As shown in Figure 2A, Hep markedly enhances the efficiency of cellular uptake of PF4 in a Hep-dependent manner. Increased number of intracellular vesicles containing labeled PF4/Hep-FITC was noted at Hep-FITC concentration of 0.25-1 U/mL (Figure 2B; fluorescent vesicles/cell: 0.6 ± 0.22 for 0.35 U/mL and 0.5 ± 0.26 for 1U/mL) as compared to PF4 alone (25 µg/mL; number of fluorescent vesicles/cell: 0.35 ± 0.07). On examining the uptake of ULCs by other phagocytic cells, we could not demonstrate PF4/Hep uptake by neutrophils, suggesting that only monocytes/DCs provide clearance of complexes. Cellular uptake of Hep-containing ULCs was not limited to complexes of PF4 and heparin but also other positively-charged proteins, as intracellular complexes could be demonstrated when Hep-FITC was incubated with murine PF4, protamine, or lysozyme to form corresponding protein/Hep-FITC ULCs. This uptake was an active process of monocytes as PF4/Hep ULC endocytosis was inhibited by 4C, and cytochalasin D, an actin polymerization inhibitor and was associated with cellular activation and expression of MHC II and CD83 co-stimulatory molecules as shown by flow cytometry (Figure 3). Finally, co-staining with KKO and lysosomal associated membrane protein-2 (LAMP-2) localized intact PF4/Hep ULCs into late endosomes. Taken together, these studies demonstrate that PF4/Hep and other protein/heparin ULCs are taken up actively by monocytes and/or DCs, intact ULCs can be detected in late endosomes and uptake is accompanied by cellular activation. These studies establish a distinct role for heparin in increasing the uptake and cellular activation of PF4 and other positively charged complexes. These studies additionally provide insights into why the majority of clinical cases of HIT occur in the wake of heparin exposure.
Arepally:TEVA Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal