Abstract
Introduction: Lenalidomide is approved by the FDA for treatment of transfusion-dependent, lower risk, deletion 5q (del(5q)) MDS patients and used widely in practice for non-del(5q) MDS patients with anemia. Recently, subsequent primary malignancies (SPM) have been reported to be associated with lenalidomide treatment of multiple myeloma, and it is unclear if this observation is disease-specific or more broadly related to a particular therapy. The SPM risk in lenalidomide-treated MDS patients has not been evaluated previously. To investigate whether lenalidomide is associated with an increased risk of SPM in MDS patients, we conducted a large, retrospective cohort study of 1,248 MDS patients treated with or without lenalidomide at the Moffitt Cancer Center (MCC).
Methods: Patients treated for MDS at MCC in 2004-2012 were identified through MCC's enterprise wide data warehouse which combined clinical information from a variety of sources, including the Cancer Registry, electronic medical records and disease-specific databases. A total of 1,248 MDS patients, ages 18+ years, were identified, corresponding to International Classification of Diseases for Oncology Third Edition (ICD-O-3) codes 99801, 99803, 99833, 99843, 99853, 99863, 99873, 99891 and 99893. A total of 41 cases of SPM were verified by two hematologists for confirmation of both the baseline MDS diagnosis and the SPM diagnosis. SPM incidence rates were estimated based on the Poisson distribution. Cox proportional hazards ratios (HR) and 95% confidence intervals (CI) were calculated to estimate the age-adjusted association between lenalidomide treatment and SPM in the overall cohort, and stratified by lower versus higher risk IPSS. To obtain additional details on lenalidomide treatment and potential confounders, medical chart abstraction was conducted for all SPM cases in addition to a sample of MDS patients from the baseline cohort who had not developed SPM; these controls were matched to cases 1:1 on age at MDS diagnosis (<60 versus 60+ years), gender, follow-up time (+/- 6 months), date of diagnosis, (+/- 1 year), lower versus higher risk IPSS, and presence or absence of del (5q). Based on the medical record data abstracted for the nested case-control sample, associations between lenalidomide and SPM were estimated using odds ratios (OR) and 95% CI's calculated through conditional logistic regression, with adjustment for age at diagnosis, use of erythroid-stimulating agents (ESA), use of azacitidine and MDS histology.
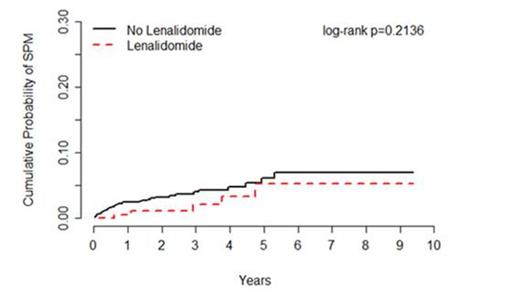
Results: Overall, 1,248 MDS patients were followed for an average of 30 months, including patients treated with (n=210) or without (n=1,038) lenalidomide. Incident SPM's were observed for 5 patients treated with lenalidomide (0.7 per 100 person-years) and 36 patients treated without lenalidomide (1.4 per 100 person-years), corresponding to an age-adjusted HR of 0.53 (95% CI=0.21-1.36) (Figure 1). Of the 41 SPM's observed, 33 were solid tumors comprised of 15 types, and 8 were hematological malignancies other than AML; no differences in SPM risk were observed by type of SPM. When stratified by IPSS, there was no increased risk of SPM observed for patients with low risk or intermediate-1 MDS (HR=0.36, 95% CI=0.11-1.20) nor for patients with intermediate-2 and high risk MDS (HR=2.30, 95% CI=0.45-11.65). Of the 41 SPM cases and 41 matched controls included in the nested case-control analysis, 12.2% (n=5) and 29.3% (n=12) were treated with lenalidomide, respectively, corresponding to an adjusted OR of 0.03 (95% CI=0.01-0.63). Similar associations were observed for lenalidomide whether given as part of first line treatment or subsequent therapy, and for lenalidomide given alone or in combination with other drugs.
Conclusion: To our knowledge this is the first report to address rate of SPM among MDS patients treated with lenalidomide. SPM was not associated with lenalidomide treatment among a large cohort of patients with a broad spectrum of MDS diagnoses.
Incidence of subsequent primary malignancies (SPM) among patients treated for myelodysplastic syndrome (MDS) with or without lenalidomide, Moffitt Cancer Center 2004-2012
Incidence of subsequent primary malignancies (SPM) among patients treated for myelodysplastic syndrome (MDS) with or without lenalidomide, Moffitt Cancer Center 2004-2012
Rollison:Celgene, Inc.: Research Funding. Off Label Use: Lenalidomide for the treatment of non-del(5q) MDS and/or multiple myeloma. Shain:Envision/Celgene: Research Funding, Speakers Bureau; L&M Healthcare/Onyx/Amgen: Research Funding, Speakers Bureau. Lee:Celgene, Inc.: Research Funding. Hampras:Celgene, Inc: Research Funding. Fisher:Celgene, Inc: Research Funding. Al Ali:Celgene, Inc: Research Funding. Padron:Icyte: Speakers Bureau; Novartis: Speakers Bureau. Lancet:Celgene: Consultancy, Research Funding. Olesnyckyj:Celgene: Employment, stock options Other. Kenvin:Celgene: Employment, stock options Other. Knight:Celgene, Inc: Employment, stock options Other. Dalton:Genentech: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene, Inc.: Research Funding. List:Celgene, Inc.: Consultancy. Komrokji:Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal