Abstract
Background: The aggressive approach to first-line treatment of multiple myeloma (MM) incorporating autologous stem cell transplantation (ASCT) remains widely prevalent, although it is not without controversy in the current era of novel effective agents. Some trials and meta-analysis comparing ASCT to non-myeloabaltive standard therapy or delayed ASCT have failed to show an overall survival (OS) difference between the two arms [Fermand et al. Blood 92:3131-3136 (1998); Koreth et al. BBMT 13:183-196 (2007); Kumar, et al. Cancer 118(6):1585-92 (2012)]. On the other hand, analysis of transplant-eligible patients receiving lenalidomide and dexamethasone induction on the E4A03 trial and then either undergoing ASCT or continuing lenalidomide and dexamethasone showed that ASCT conferred improved OS (Blood 2010;116:38a). This controversy lead us to design a phase II clinical trial comparing continuous lenalidomide and dexamethasone (Ld) versus ASCT followed by lenalidomide maintenance, in patients responding to four cycles of Ld.
Methods: Patients with newly diagnosed symptomatic MM as defined by IMWG criteria were enrolled. Patients deemed to be in urgent need of aggressive therapy (e. g. symptomatic bone disease, acute renal failure, hyperviscosity syndrome, etc ) were not eligible. Patients received induction with lenalidomide (L) 25 mg PO daily on days 1-21, and dexamethasone (d) 40 mg PO daily on days 1,8,15, and 22 of a 28-day cycle with standard prophylaxis. Patients with POD during induction or SD after four cycles of Ld were taken off study. All other patients had stem cells harvested after four cycles of Ld and were randomized to either the continuous (Ld) arm (L at the last tolerated dose during induction, continued indefinitely until progression or toxicity; and d at 20mg weekly for one year) or the ASCT arm (using melphalan conditioning followed by L maintenance started three months post-ASCT at 10mg daily, escalated to 15 mg daily six months post-ASCT and continued indefinitely until progression or toxicity).
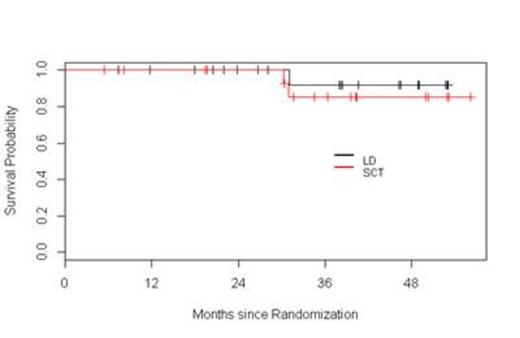
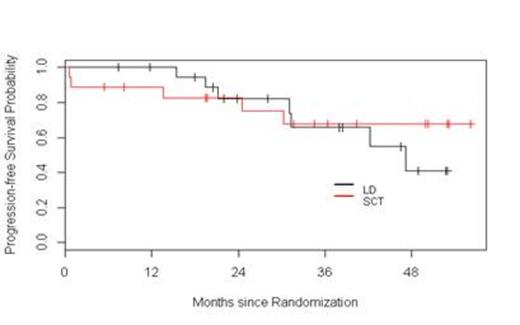
Results: Fifty seven patients have been registered to the trial. Two patients did not initiate therapy, one because of a concurrent diagnosis of amyloidosis and the other due to aggressive disease mandating alternative therapy according to the treating physician. At this time, four patients are still receiving induction therapy and are not available for response assessments. Among the 51 remaining patients, the response to initial induction therapy includes 12% CR (n=6), 2% uCR (n=1), 4% nCR (n=2), 14% VGPR (n=7), 51% PR (n=26), 6% SD (n= 3), 4% POD (n=2), and 8 % inevaluable (n = 4 who did not receive at least two cycles of Ld). Thirteen patients were removed from the trial prior to randomization due to POD (n=2), SD after four cycles (n=3), toxicity (n=4), physician discretion (n=2), and withdrawal of consent (n=2). Among these 13 patients, one was lost to follow-up, two continued lenalidomide therapy off protocol (one patient refused ASCT, and one patient had inadequate stem cell collection), and 10 proceeded to alternative induction. All 12 patients achieved a response [25 % CR (n=3), 42 % VGPR (n=5), and 33 % PR (n=4)]; 10 patients proceeded to ASCT without event. Thirty-eight patients were randomized, 20 to Ld and 18 to ASCT. Improvement of response by at least one level occurred in 45% and 65% of patients on the Ld and ASCT arms, respectively. The median follow-up for all surviving patients from time of randomization is 38.3 months. The 1- and 3-year PFS in the Ld arm were 100% and 66%, respectively. The 1- and 3-year PFS in the ASCT arm were 89% and 68%, respectively. The 2- and 3-year OS in the Ld arm were 100% and 92%, respectively. The 2- and 3-year OS in the ASCT arm were 100% and 85%, respectively. Considering the entire population of 51 patients, with a median follow-up of 38 months, the median PFS has not been reached.
Conclusions: This interim analysis, with relatively short follow-up, suggests that in transplant-eligible patients responsive to Ld during induction, continuous Ld results in similar PFS and OS compared to patients who undergo ASCT followed by L maintenance. Furthermore, this overall approach based on response-adapted treatment results in 100% of patients reaching at least PR at completion of first-line therapy. The trial remains ongoing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal