Abstract
Relapse is the leading cause of death following allogeneic hematopoietic cell transplant (HCT) for high risk acute myeloid leukemia (AML). Antigen-specific T cells that selectively target leukemia associated antigen (LAA) have the potential to promote graft versus leukemia (GVL) activity without inducing graft versus host disease (GVHD). We previously demonstrated that transferred donor derived WT1-reactive CD8+ cytotoxic T-cells (CTL) clones can persist in post-transplant patients and mediate anti-leukemic activity (Chapuis et al. Sci Transl Med 2013; 5, 174ra27).
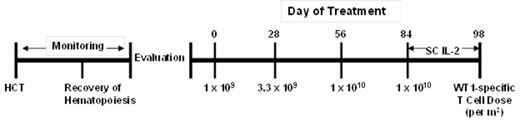
T cell receptor (TCR) gene transfer offers the potential to reproducibly provide every patient with a high avidity T cell response. In this study, escalating doses of donor-derived virus specific CD8+ T cells transduced to express a high affinity TCR specific for the HLA A*02:01-restricted WT1126-134 (RMFPNAPYL) epitope are being administered to high-risk AML patients after allogeneic HCT, with escalating doses withheld until persistence of the previous dose is at a frequency of ≤3% of peripheral blood CD8+ T cells. Currently, 9 patients have been treated on study and received a total of 22 infusions. Three patients have completed the four T cell infusions, with the last infusion followed by a two week course of Interleukin 2 (Fig.1). CTC Grade ≥ 3Adverse Events have been transient hypotension and a febrile reaction, and transient leukopenia, lymphopenia, and thrombocytopenia. No end-organ toxicities attributed to the infused T cells have been observed. One patient experienced exacerbation of acute GVHD after T cell infusions, and one patient developed chronic GVHD, although there is currently no evidence the GVHD in either patient reflected activity of the infused T cells.
Three patients, who were treated with T cells after a second allogeneic HCT for relapsed AML (two of whom had persistent/relapsed disease after second HCT), are alive with no evidence of disease 14, 8 and 7 months after initiation of T cell infusion (16, 26, and 9 months after second transplant) with no additional anti-leukemic therapy after completion of study treatment. One patient with high risk AML was treated prophylactically after allogeneic HCT for AML in second complete remission (CR2), and is alive with no evidence of disease 13 months after initiation of study treatment (15 months after transplant) (Table 1). Transferred CTL were detectable between 4 and >290 days after infusion (Table 1, Fig.2).
The study continues to accrue new patients, but the preliminary results suggest that transfer of donor-derived virus specific CD8+ T cells transduced to express a WT1-specific TCR can be accomplished without significant toxicity and that such therapy can potentially provide anti-leukemic activity. Additionally, no immunogenicity of transferred cells has been observed, suggesting the possibility of retreatment at later time points in case of subsequent relapse.
Clinical Outcomes
| Patient . | Diagnosis . | Disease status prior to study treatment . | Disease burden during T cell infusion . | Number of infusions . | CTL persistence (days after last infusion) . | Outcome* . | Survival* . |
|---|---|---|---|---|---|---|---|
| 1 | AML | Relapse 5 years after allogeneic HCT (medullary and extramedullary disease) | Present | 3 | 14+ | Progressive disease | Alive |
| 2 | AML | Relapse 10 years after first allogeneic HCT. MRD early after second HCT | Present | 4(+IL2) | 290+ | Remission 16 months after transplant | Alive |
| 3 | AML | HCT at CR2. No evidence of disease after HCT | Absent | 4(+IL2) | 20 | Remission 15 months after transplant | Alive |
| 4 | AML | Relapse with extramedulary disease one year after second allogeneic HCT | Absent | 1 | 210+ | Remission 26 months after transplant (8 months after treatment) | Alive |
| 5 | MDS → AML | Persistent disease after HCT | Present | 1 | 5+ | Progressive disease | Dead |
| 6 | AML | Second HCT for relapse 4 years after first HCT | Absent | 4(+IL2) | 4 | Remission 9 months after transplant | Alive |
| 7 | AML | Persistence disease after HCT | Present | 1 | 30+ | Progressive disease | Dead |
| 8 | AML | HCT in CR2. MRD early after transplant | Present | 1 | 50+ | Ongoing treatment | Alive |
| 9 | AML | HCT in CR2. Relapse early after transplant | Absent | 3 | 14+ | Ongoing treatment | Alive |
| Patient . | Diagnosis . | Disease status prior to study treatment . | Disease burden during T cell infusion . | Number of infusions . | CTL persistence (days after last infusion) . | Outcome* . | Survival* . |
|---|---|---|---|---|---|---|---|
| 1 | AML | Relapse 5 years after allogeneic HCT (medullary and extramedullary disease) | Present | 3 | 14+ | Progressive disease | Alive |
| 2 | AML | Relapse 10 years after first allogeneic HCT. MRD early after second HCT | Present | 4(+IL2) | 290+ | Remission 16 months after transplant | Alive |
| 3 | AML | HCT at CR2. No evidence of disease after HCT | Absent | 4(+IL2) | 20 | Remission 15 months after transplant | Alive |
| 4 | AML | Relapse with extramedulary disease one year after second allogeneic HCT | Absent | 1 | 210+ | Remission 26 months after transplant (8 months after treatment) | Alive |
| 5 | MDS → AML | Persistent disease after HCT | Present | 1 | 5+ | Progressive disease | Dead |
| 6 | AML | Second HCT for relapse 4 years after first HCT | Absent | 4(+IL2) | 4 | Remission 9 months after transplant | Alive |
| 7 | AML | Persistence disease after HCT | Present | 1 | 30+ | Progressive disease | Dead |
| 8 | AML | HCT in CR2. MRD early after transplant | Present | 1 | 50+ | Ongoing treatment | Alive |
| 9 | AML | HCT in CR2. Relapse early after transplant | Absent | 3 | 14+ | Ongoing treatment | Alive |
* as of 7/10/14
+ persistence T cells detected at most recent analysis
Figure 1. Treatment plan
Figure 2. In vivo persistence of WT1-specific donor derived transduced CTL
Greenberg:Juno Therapeutics: Consultancy, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal