Abstract
The therapeutic benefit of allogeneic hematopoietic stem cell transplantation (allo-HSCT) for treatment of hematologic malignancies is primarily derived from the anti-leukemia effect mediated by T cells contained in the donor graft. Unfortunately, these T cells also mediate graft-versus-host disease (GvHD), the major complication of allo-HSCT. We and others have demonstrated that interferon gamma receptor deficient (IFNγR-/-) allogeneic donor grafts, when transplanted into wild-type (WT) recipients (IFNγR-/- donors → WT recipients), reduce GvHD compared with WT donor grafts (WT donors → WT recipients) whilst maintaining a robust Graft vs. Leukemia effect (GvL).
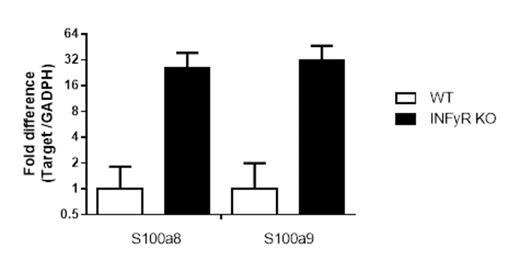
The aim of the present study was to elucidate the mechanism by which IFNyR deficient donor T cells reduce GvHD. To achieve this we performed RNA expression analyses on allo-activated T cells. To obtain allo-activated T cells, we co-cultured B6 CD4+CD25- T cells, isolated from WT and IFNγR-/- mice, with irradiated Balb/c antigen presenting cells (APCs). After six days of co-culture, CD4+CD25+ allo-activated T cells were sorted and total RNA extracted. Subsequent RNA profiling was performed using Mouse Genome 430 2.0 array (Affymetrix). From the numerous genes found to be differentially regulated between WT and IFNyR-/- T cells we sought to identify, for further analysis, those genes meeting the following criteria: (i.) genes with known human orthologs, (ii.) genes exhibiting the most pronounced differential expression between WT and IFNγR-/- T cells, (iii.) genes for which knockout or transgenic mice are available and (iv) genes with products that can be targeted by small molecule inhibitors. Two candidates were identified by applying all mentioned criteria: S100A8 and S100A9. Expression levels of S100A8 and S100A9 were 22 and 26 fold higher in APC activated IFNγR-/- T cells than WT T cells, respectively, which was also confirmed using qRT-PCR (Figure 1).
It has been reported that S100A8 and S100A9, two members of the calgranulin family of proteins, form a heterodimer complex and may modulate/mitigate several inflammatory diseases by potentially binding NFKB intracellularly and inflammatory cytokines such as IL-6 and TNF when excreted extracellularly.1,2 Overexpression of S100A9 alone is sufficient to upregulate the expression of S100A8 and vice versa.3 In addition, S100A9-/- mice also lack expression of S100A8.4 We hypothesized that S100A8/S100A9 expression in allo-reactive donor T cells functions as a suppressor of GvHD. To test this hypothesis, we performed allo-HSCT with donor T cells isolated from either S100A9 overexpressing transgenic or WT B6 mice transplanted into Balb/c recipients. S100A9 overexpressing donor T cells induced significantly less GvHD than WT T cells. Recipients of S100A9 overexpressing T cells survived significantly longer (p=0.049, n=5), had reduced weight loss and higher percentages of B220+ B cells (indicative of less severe clinical GvHD) than recipients of WT T cells. This suggests that S100A9 might indeed function as a suppressor of GvHD and could account, at least in part, for the diminished GvHD potential of IFNyR-/- T cells. Modulation of the S100A9/A8 complex therefore represents a novel therapeutic target for the treatment of GvHD.
The relative expression of S100A8 and S100A9 in allo-activated T Cells determined by qRT-PCR. IFNyR -/- vs. WT APC activated T cells.
The relative expression of S100A8 and S100A9 in allo-activated T Cells determined by qRT-PCR. IFNyR -/- vs. WT APC activated T cells.
1. Otsuka K, Terasaki F, Ikemoto M, et al. Suppression of inflammation in rat autoimmune myocarditis by S100A8/A9 through modulation of the proinflammatory cytokine network. Eur J Heart Fail. 2009;11(3):229-237.
2. Ikemoto M, Murayama H, Itoh H, Totani M, Fujita M. Intrinsic function of S100A8/A9 complex as an anti-inflammatory protein in liver injury induced by lipopolysaccharide in rats. Clin Chim Acta. 2007;376(1-2):197-204.
3. Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205(10):2235-2249.
4.Manitz MP, Horst B, Seeliger S, et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants invitro. Mol Cell Biol. 2003;23(3):1034-1043.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal