Abstract
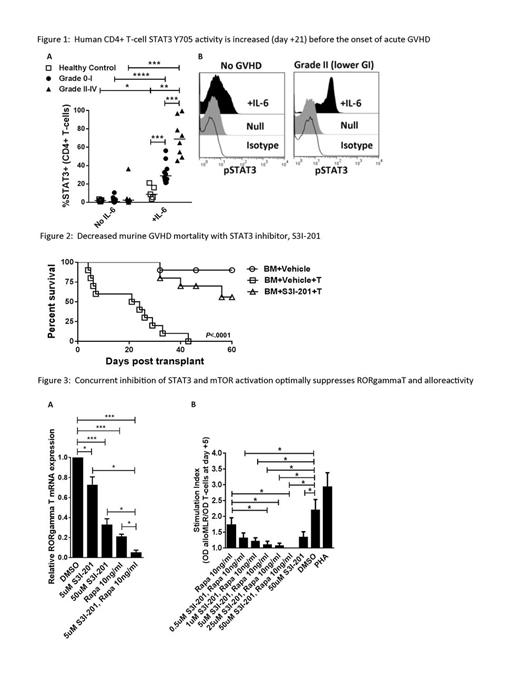
STAT3 is a key mediator of inflammation, and is associated with murine auto- and alloreactivity. Germline and somatic activating STAT3 mutations have correlated with human inflammatory disorders, including colitis and polyendocrinopathy. STAT3 phosphorylation is required for pathogenic Th17 differentiation, and antagonizes beneficial Treg development. We show that CD4+ T-cell STAT3 activity is elevated early after allogeneic hematopoietic cell transplantation (HCT) compared with healthy non-HCT controls, and identifies patients at risk of developing grade II-IV acute graft-versus-host disease (GVHD) before the onset of symptoms. Using human in vitro and murine in vivo systems, we demonstrate that STAT3 phosphorylation may be selectively targeted with S3I-201 to suppress the alloresponse while preserving graft-versus-leukemia (GVL). Peripheral blood T-cells were obtained on day +21 from consented HCT recipients lacking any signs of acute GVHD (n=5 non-HCT controls, n=18 HCTs). T-cell STAT3 Y705 activity was stimulated with a pulse of IL-6 (4000 IU/ml, 15 minutes) and measured by flow cytometry. Patients were followed for acute GVHD onset from day +22 to day +100. While resting STAT3 activity was very low in all groups, stimulated CD4+ STAT3 phosphorylation significantly increased among the patients that later developed acute GVHD during the observation period (Fig 1 A: non-HCT control: 8.83%; n=10 grade 0-I: 28.8%; n=8 grade II-IV: 67.6% median pSTAT3+/CD4+; P<.05 and <.001; Fig 1 B: representative histograms show day +21 STAT3 activity in a patient that never acquired GVHD v a patient that later developed grade II GVHD at day +43). The absolute number of pSTAT3+ CD4+ T-cells on day +21 was also increased among the patients that acquired grade II-IV acute GVHD (grade 0-1: 45.5; grade II-IV: 83.5 CD4+ T-cells/ul; P<.05). The total absolute number of CD4+ T-cells, and number and degree of pSTAT3+ CD8+ T-cells, was similar among all HCT groups. We previously demonstrated that S3I-201 suppressed human DC-allostimulated T-cells, selectively polarized STAT5 activity while inhibiting STAT3, abrogated Th17 responses, and permitted the expansion of potent antigen-specific inducible Treg (Betts et al, Journal of Leukocyte Biology, 2014). We now show S3I-201 prevents GVHD in vivo, where Balb/c mice received myeloablative irradiation (850 cGy) followed by an allograft of T-cell depleted or replete (1x10e6) C57BL/6 bone marrow (5x10e6). S3I-201 (2.5mg/kg or DMSO control) was given i.p. from day 0 to +7. S3I-201 significantly delayed GVHD onset and improved survival (Fig 2, 10 mice/group, 0% v 56% alive at day +60, P<.0001, n=2). To determine the effect of S3I-201 on GVL, similarly transplanted Balb/c mice additionally received 2000 luciferase-expressing A20 cells. In this pilot experiment, median survival for the vehicle-treated BM-A20 and T cell-A20 groups was 26 days, 40.5 days for S3I-BM-A20, and not reached for S3I-T cell-A20 (67% alive at day +60). Bioluminescence (IVIS 200) remained undetectable in both the T cell-A20 and S3I-T cell-A20 groups during the experiment. Moreover, as mTOR and STAT3 signaling both influence Th17 differentiation we show that concurrent exposure of human T-cells to rapamycin and S3I-201 optimally suppresses RORgammaT expression and enhances allo-inactivation (Fig 3 A: P<.05, <.001, n=3, Fig 3 B: colorimetric proliferation assay, DC:T 1:30, P<.05, n=4). Ongoing experiments are underway to study the effect of combined rapamycin and S3I-201 on GVHD and GVL in vivo. Our preclinical data implicates STAT3 activation early in the onset of acute GVHD, where its selective inhibition with S3I-201 controls alloreactivity without untoward impairment of Treg or GVL. Future investigation of this approach will incorporate specific STAT3 inhibition with rapamycin to develop novel GVHD prevention strategies, and validate the association of STAT3 activity with acute GVHD risk in a large cohort of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal