Abstract
Background: Childhood acute myeloid leukemia (AML) remains a challenging disease as the outcome is still poor despite major improvement over the past decades; current survival rates are around 70% but event free survival (EFS) is only about 50%. No benefit of standard maintenance chemotherapy has been proven after intensive induction/consolidation chemotherapy.
Objective: To determine whether the addition of a one-year maintenance therapy using interleukin-2 (IL-2), known to stimulate antitumor immunity, decreases the risk of relapse and improves EFS in pediatric AML.
Methods: ELAM02 trial was designed to recruit patients aged from 0 to 18 years, diagnosed with primary AML. Children with acute promyelocytic leukemia and Down Syndrome were not included. The treatment consisted of one induction course (cytarabine and mitoxantrone) and three consolidation courses (course 1 and 3 with high dose cytarabine); all children without t(8;21) were candidates for hematopoietic stem cell transplant (HSCT) in complete remission (CR) after 1 to 2 courses of consolidation if a geno-identical donor was available; children with poor-prognosis karyotype were also candidates for HSCT with pheno-identical donor.
The patients not receiving HSCT and in continuous CR after the third course of consolidation were eligible for randomization for a one-year maintenance therapy consisting in monthly courses of IL-2. IL-2 (Proleukin®, Chiron, Novartis) was given subcutaneously at 2.5 MUI/m² on day 1 and at 5 MUI/m² from day 2 to 5. Cycles were planned to be given monthly for up to 12 cycles. In case of side effects such as severe (grade ≥ 3) clinical toxicities (fever >40°C, hypotension requiring IV fluids) and/or severe biological toxicities (thrombocytopenia (grade ≥ 3), renal dysfunction (grade ≥ 2), liver dysfunction (grade ≥ 3)) doses of IL-2 was lowered of 50%. In case of persistent side effects, treatment was discontinued. The control group received no maintenance treatment.
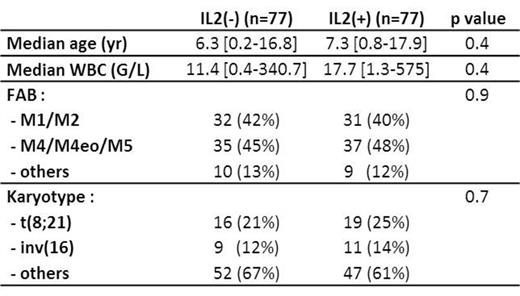
Results: The 28 French SFCE centers participated to the study, leading to the enrollment of 441 patients from March 2005 to December 2011. Among the 441 enrolled patients, 3 patients were excluded due to non-conformity of inclusion criteria; 392/438 (89%) were in CR after the first consolidation course and 116 (30%) were allografted in CR1. Out of the 241 eligible patients for randomization, i.e. still in CR after the third course of consolidation, 154 (64%) were actually randomized for maintenance therapy; causes for non-randomization were either parents refusal (n=50, 21%) or medical decision (n=37, 15%). Median follow-up is 5 years. The characteristics of the randomized patients at diagnosis were as follows:
Median number of IL-2 cycles administered was 12 [5-12], the mean being 8.6 ± 4.2. Among the 77 patients receiving maintenance therapy, IL-2 was stopped before cycle 6 in 20 patients (26%) and after cycle 6 in 18 (23%); 39 patients (51%) received 12 cycles. Treatment was stopped because of relapse occurrence (n=15), severe persistent toxicities (n=6) or parents or medical decision (n=17). The most frequent toxicities related to IL-2 treatment were fever, chills, and cytolytic hepatitis; no toxic death related to IL-2 therapy was observed. Incidence of relapses in IL2+ group and IL2- group were 36% (n=28) and 38% (n=29) respectively. The 5-year disease free survival (DFS) was 62 % (95% CI 51-73) for the IL2- group vs. 64% (95% CI 53-75) for the IL2+ group (p=0.74). Among the CBF population, a trend in favor of the IL-2 treatment was observed as the 5-year DFS was 57% (95% CI 43-71) for the IL2- group vs. 78% (95% CI 63-94) for the IL-2+ group (p=0.08).
Conclusion: A prolonged administration of IL-2 as maintenance therapy after intensive chemotherapy is feasible in pediatric AML patients in first CR but did not improve DFS in this study.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal