Abstract
Internal tandem duplication mutations of the FLT3 tyrosine kinase (FLT3-ITDs) are present in 15-30% of cases of pediatric and adult acute myeloid leukemias (AML), and are known to portend a poor prognosis. Over the past decade small molecule inhibitors targeting FLT3 have entered clinical trials. Although initial responses have been observed, patients typically develop resistance to current FLT3 inhibitors during the first year of therapy via acquisition of or selection for point mutations in the FLT3 kinase domain at amino acid F691 or D835. Although FLT3 is the most commonly targeted protein in AML, other candidates for pharmacologic inhibition have been identified. Ectopic expression of MerTK, a receptor tyrosine kinase, has been identified in 80-100% of primary AML patient samples, and previous data demonstrate anti-leukemic effects in response to shRNA-mediated MerTK inhibition. Here we describe MRX2843, a novel small molecule inhibitor of MerTK and FLT3 with activity against FLT3 point mutations and therapeutic efficacy in mouse xenograft models of AC220-resistant AML.
MRX2843 potently inhibits MerTK and FLT3 with enzymatic IC50 values of 1.3 and 0.64nM, respectively. Treatment of two AML cell lines that express a FLT3-ITD mutation (MV4;11 has low MerTK expression; MOLM-14 does not express MerTK), and two AML cell lines that express MerTK but do not have activating mutations in FLT3 (U937, Kasumi-1) with 25-300nM MRX2843 abrogated activation of intracellular signaling pathways downstream of FLT3 and MerTK, including AKT and ERK1/2. In addition, 72 hour treatment with MRX2843 led to induction of apoptosis in AML cell lines compared to vehicle-treated controls, as determined by flow cytometic analysis after staining with YO-PRO-1 iodide and propidium iodide. For example, in MOLM-14 cultures, treatment with MRX2843 resulted induction of apoptosis in 84±2% of cells, compared to 5±2% after vehicle treatment (p<0.001). In soft agar or methylcellulose cultures, treatment of AML cell lines and patient samples with MRX2843 resulted in an 80-90% reduction in colony number.
To determine the effects of MRX2843 treatment in vivo, a patient-derived murine xenograft model was established by intravenous injection of primary AML patient blasts that express both MerTK and FLT3-ITD into NOD-SCID-gamma (NSG) mice. Engraftment was monitored by flow cytometric determination of peripheral blast count. When ~10% peripheral blasts were detected, mice were randomized to treatment with 50mg/kg MRX2843 or vehicle (saline) once daily by oral gavage. Treatment with MRX2843 significantly prolonged survival (median survival of 96 days after MRX2843 treatment versus 16 days in control mice, n=4 per group, p<0.01).
Two derivatives of the human FLT3-ITD AML cell line MOLM-14, which acquired either the D835Y (activation loop) or F691L (gatekeeper) mutation after selection in escalating doses of the FLT3 inhibitor AC220, were used to test the activity of MRX2843 against clinically relevant FLT3 point mutations. Treatment with MRX2843 resulted in a significant reduction in the number of viable cells after 48 hours of culture in MOLM-14, MOLM-14:D834Y, and MOLM-14:F691L with IC50 values of 17nM, 20nM, and 30nM, respectively. In both mutant cell lines, MRX2843 potently inhibited phosphorylation of FLT3 and abrogated activation of downstream intracellular signaling molecules. Treatment with MRX2843 induced cell death in MOLM-14:D835Y (80±7% versus 10±1%, p<0.001) and in MOLM14:F691L (61±16% versus 12±1%, p<0.001). In contrast, both cell lines were resistant to treatment with AC220 at concentrations 20-fold higher than the inhibitory concentration in the parental line.
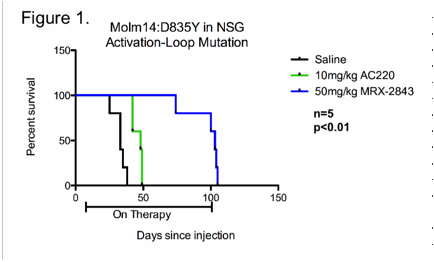
A murine model of AC220-resistant AML was developed by intravenous injection of MOLM-14:D835Y cells into NSG mice. Daily treatment with saline, 10mg/kg AC220, or 50mg/kg MRX2843 was administered by oral gavage beginning 4 days after transplant. Treatment with MRX2843 significantly prolonged survival when compared to mice treated with saline or AC220 with median survival of 103 days, 33 days, and 48 days, respectively (n=5 per group, p<0.01, Figure 1).
In summary, MRX2843 is a novel MerTK and FLT3 inhibitor that retains activity against clinically relevant, resistance-conferring FLT3 point mutations and prolongs survival in murine models of AML. These data support further development of MRX2843 and advancement to early phase clinical trials.
DeRyckere:University of Colorado - Denver: targeting of the Mer tyrosine kinase as cancer therapy Patents & Royalties; Meryx, Inc: Equity Ownership. Wang:University of North Carolina Chapel Hill: MRX-2843 Patents & Royalties; Meryx, Inc: Equity Ownership. Frye:University of North Carolina Chapel Hill: MRX-2843 Patents & Royalties; Meryx, Inc: Equity Ownership. Earp:University of North Carolina Chapel Hill: MRX-2843 Patents & Royalties; University of North Carolina Chapel Hill: targeting of the Mer tyrosine kinase as cancer therapy Patents & Royalties; Meryx, Inc: Equity Ownership. Graham:University of Colorado - Denver: MRX-2843 Patents & Royalties; University of Colorado - Denver: targeting of the Mer tyrosine kinase as cancer therapy Patents & Royalties; Meryx, Inc: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal