Abstract
Background: Blinatumomab is an investigational bispecific T-cell engager (BiTE®) antibody construct designed to direct cytotoxic T cells to CD19-expressing B cells. In a study in minimal residual disease (MRD)-positive ALL, 80% of pts achieved MRD-negative status after receiving one treatment cycle of blinatumomab (Topp MS, et al. J Clin Oncol. 2011;29:2493-8). For pts with newly diagnosed ALL, persistence of MRD after first-line treatment predicts disease recurrence. However, only limited data are available about the relationship between MRD and outcome in adult pts with r/r ALL. Therefore, the aim of this secondary analysis was to evaluate MRD responses and their relationships to other efficacy indices in adult pts with r/r ALL receiving blinatumomab in this phase 2 study.
Methods: Eligibility criteria included adult pts ≥18 years, with Philadelphia chromosome negative B-cell r/r ALL who were primary refractory or refractory to first salvage, had their first relapse within 12 months of first remission or allogeneic hematologic stem cell transplantation (HSCT), or received second or later salvage. Blinatumomab was dosed via continuous IV infusion at 28 μg/d (cycle 1 only: 9 μg/d on days 1-7, 28 μg/d on days 8-28) for up to five cycles (one cycle: 4 weeks on, 2 weeks off). The primary endpoint was complete remission (CR: ≤5% blasts with platelets >100,000/µL, absolute neutrophil count >1000/µL) or CR with partial hematologic recovery (CRh*: platelets >50,000/µL, absolute neutrophil count >500/µL) within the first two treatment cycles. MRD in bone marrow was assessed at a central laboratory using allele-specific real-time quantitative PCR. Two MRD-based exploratory endpoints were analyzed: MRD response (no PCR amplification at a minimum sensitivity of 10-4 or <10-4 leukemic cells by PCR) and complete MRD response (no PCR amplification at a minimum sensitivity of 10-4) within the first two treatment cycles.
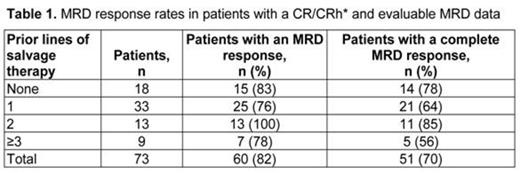
Results: Of 189 treated pts (median [range] age = 39 [18–79] years), 81 (43%) pts had a CR (n = 63, 33%) or a CRh* (n = 18, 10%) during the first two treatment cycles. In the subgroup of pts with a CR/CRh* and evaluable MRD data (n = 73), 60 (82%) pts had an MRD response. Of those, 51 (70%) pts had a complete MRD response. The majority of those who achieved a CR/CRh* also achieved a complete MRD response regardless of the number of prior lines of salvage therapy (Table 1). Of all treated pts, 64 (34%) pts had prior HSCT. In pts who achieved CR/CRh*, the rate of MRD response was 81% in pts without and 85% in pts with prior HSCT. In pts who achieved CR/CRh*, the median (95% CI) duration of overall survival was 11.4 (8.5, not estimable) months for those with a MRD response and 6.7 (2.0, not estimable) months for those with no MRD response. The median (95% CI) duration of relapse-free survival was 6.9 (5.5, 10.1) months in patients with a MRD response and 2.3 (1.2, not estimable) months in patients with no MRD response. The HSCT realization rate did not differ significantly between pts with CR/CRh* and a MRD response and those with CR/CRh* and no MRD response (31% and 43%, respectively).
Seventeen pts classified as nonresponders per protocol had ≤5% blasts and no evidence of disease, but recovery of peripheral counts did not meet criteria for CRh*. Ten of these 17 pts had evaluable MRD assessments; 50% (5/10) had MRD response and 20% (2/10) had complete MRD response. The clinical significance of this finding remains open.
Grade ≥3 adverse events regardless of causality and occurring in >10% of all treated pts during treatment until 30 days after treatment were febrile neutropenia (25%), neutropenia (16%), and anemia (14%). Grade 3 cytokine release syndrome (CRS) was reported in three (1.6%) patients. Three (1.6%) patients had grade 4 neurologic events, all resolved. Twenty-one (11%) patients had grade 3 neurologic events, 17 were resolved, with 4 events unresolved at death or unknown.
Conclusion: In this largest study to date of adult pts with r/r ALL with PCR-based central assessment of MRD, pts who achieved CR/CRh* during single-agent treatment with blinatumomab had high MRD response rates. The exploratory analyses suggested that within pts with a CR/CRh*, those who did not achieve MRD responses tended to have shorter durations of overall and relapse-free survival. These results are consistent with prior reports highlighting the importance of achieving an MRD response in first-line therapy.
Goekbuget:Amgen Inc.: Consultancy, Honoraria, Research Funding. Off Label Use: This presentation will discuss the off-label use of blinatumomab, as this agent is not approved for use by the FDA, EMA or any other regulatory authorities. Kantarjian:Amgen Inc.: Research Funding. Brüggemann:Amgen Inc.: Consultancy, Research Funding. Stein:Amgen Inc.: Membership on an entity's Board of Directors or advisory committees. Bargou:Amgen Inc.: Consultancy, Honoraria. Dombret:Amgen Inc.: Research Funding. Heffner:Amgen Inc.: Honoraria, Research Funding; Biotest: Research Funding; Dana Farber CI: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Idera: Research Funding; Janssen: Research Funding; Pfizer: Research Funding; Pharmacyclics: Honoraria, Research Funding; Onyx: Research Funding; Spectrum: Research Funding; Talon Therapeutics: Research Funding. Rigal-Huguet:Amgen, Inc.: Consultancy; Ariad: Consultancy; BMS: Consultancy; Novartis: Consultancy; Pfizer: Consultancy. Litzow:Amgen Inc.: Honoraria, Research Funding. Zugmaier:Amgen Reseach (Munich) GmbH: Employment; Amgen Inc.: Equity Ownership. Jia:Amgen Inc.: Employment, Equity Ownership. Maniar:Amgen Inc.: Employment, Equity Ownership. Huber:Amgen Research (Munich) GmbH: Employment; Amgen Inc.: Equity Ownership. Nagorsen:Amgen Inc.: Employment, Equity Ownership, Related to blinatumomab Patents & Royalties. Topp:Amgen Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal