Abstract
Background: Acute myeloid leukemia (AML) with t(8;21) or inv(16) is commonly referred to as core-binding factor AML (CBF-AML). Although this group represents a favorable cytogenetic AML subgroup, 30-40% of these patients nevertheless relapse after standard intensive chemotherapy. The incorporation of high-dose cytarabine for postremission therapy has substantially improved the outcome of CBF-AML patients, especially when administered as 2-4 repetitive cycles. Here we present retrospective data from a single centre, on this favourable AML subgroup.
Methods: We analyzed retrospectively the outcome of 80 sequential patients with CBF-AML (46 t(8;21), 34 inv(16)/t(16;16)) treated over a 13 year period from 2000-2012. The median age was 48 years (range 20-80) with a median white cell count of 13x109/L(range 1-426x109/L). All patients underwent induction chemotherapy consisting of daunorubicin (60 mg/m2/d x 3 days) and continuous infusion cytarabine (100 or 200 mg/m2/d x 7 days, for ages <60y and ≥60y, respectively). Patients in CR then received 3 cycles of consolidation chemotherapy which included high dose cytarabine (3 or 1.5 g/m2 on days 1, 3 and 5, for ages <60y and ≥60y, respectively) with daunorubicin 45 mg/m2 on days 1 and 3 added during cycle 1.
Results: Ninety percent of patients achieved morphological complete remission (CR) post induction, but 33.3% of these remained molecularly positive by quantitative reverse transcriptase polymerase chain reaction (qR-PCR). The majority of patients (93.6%) subsequently achieved molecular negativity by qR-PCR after the 3rd consolidation cycle.
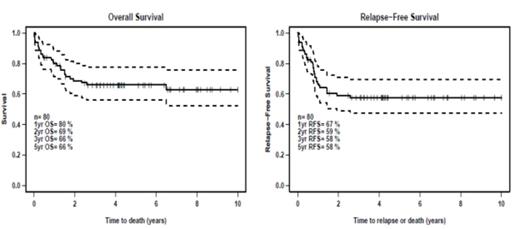
With a median follow-up of 5 years, the estimated 5-year relapse free survival (RFS) was 58% and 5-year overall survival (OS) was 66%. This is in keeping with the earlier AMLSG and UK MRC studies. Only 21% of patients relapsed and the median time to relapse was 10 months. Of the 16 patients who relapsed, 7 underwent allogeneic hematopoietic cell transplantation (HCT) from HLA- matched sibling or unrelated donors, and of these, 4 remain alive and in remission with a median follow up of 8 years.
While relapse occurred in 2 of the 4 patients who did not achieve a molecular CR after 3 cycles of consolidation, 13 of 16 relapsing patients had been in a molecular CR after final consolidation and one patient was not evaluated. Therefore, molecular remission post consolidation does not guarantee long term disease free survival. Further molecular analyses and the roles of additional mutations in predicting relapse will be presented.
Conclusion: Sustained remissions can be achieved in patients with CBF AML after 3 cycles of high dose cytarabine consolidation, but post consolidation molecular remission does not necessarily preclude relapse. Comprehensive molecular profiling may be better in predicting relapse risk in this group of patients.
Patient characteristics & response
| . | N=80 . | . |
|---|---|---|
| Sex | 45 M : 35 F | |
| Median age, years (range) | 48 (20-80) | |
| WBC at diagnosis (x106 cells/L) (range) | 13 (1-426) | |
| Cytogenetics t(8;21) inv16/t(16;16) | 46 (58%) 34 (42%) | |
| Response post-induction chemotherapy CR Primary refractory | 72 (90%) 8 (10%) | |
| Number of consolidation cycles (range) | 3 (2-4) | |
| Molecular CR post –induction Yes No Not evaluated | 16 (20%) 32 (40%) 32 (40%) | |
| Molecular CR post –consolidation –cycle 3 Yes No Not evaluated | 59 (74%) 4 (5%) 17 (21%) | |
| Relapse Yes No Unknown | 16 (20%) 59 (74%) 5 (6%) | |
| Median time to relapse, months (range) | 10 (6-17) | |
| Median time to follow up, y (range) | 5 (0.5-11.5) |
| . | N=80 . | . |
|---|---|---|
| Sex | 45 M : 35 F | |
| Median age, years (range) | 48 (20-80) | |
| WBC at diagnosis (x106 cells/L) (range) | 13 (1-426) | |
| Cytogenetics t(8;21) inv16/t(16;16) | 46 (58%) 34 (42%) | |
| Response post-induction chemotherapy CR Primary refractory | 72 (90%) 8 (10%) | |
| Number of consolidation cycles (range) | 3 (2-4) | |
| Molecular CR post –induction Yes No Not evaluated | 16 (20%) 32 (40%) 32 (40%) | |
| Molecular CR post –consolidation –cycle 3 Yes No Not evaluated | 59 (74%) 4 (5%) 17 (21%) | |
| Relapse Yes No Unknown | 16 (20%) 59 (74%) 5 (6%) | |
| Median time to relapse, months (range) | 10 (6-17) | |
| Median time to follow up, y (range) | 5 (0.5-11.5) |
Gupta:Novartis: Consultancy, Honoraria, Research Funding; Incyte Corporation: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal