Abstract
Introduction
Intensive “pediatric oriented” treatment with heavy cytostatic load, incorporation of allogeneic HSCT is now considered a backbone approach in adult ALL therapy. Highly myelosuppressive treatment is more toxic and less reproducible, so RALL has initiated non-intensive but non- interruptive protocol “ALL-2009”and started a prospective multicenter trial for adult Ph-negative ALL based on: 1) the replacement of prednisolone (Pdn) 60 mg/m2 with dexamethazone (Dexa) 10 mg/m2 if blast cells are >25% in bone marrow after 7 days of prephase; 2) de-intensified but non-interruptive treatment with doses modification according to the myelosuppression with total treatment duration of 127 weeks; 3) prolonged implication of L-asparaginase (total proposed dose 590.000 IU/m2); 4) autologous hematopoietic stem cell transplantation (HSCT) after non-myeloablative BEAM conditioning followed by prolonged maintenance – for T-cell ALL patients. Allo-BMT was an option for high risk patients with sibling donors. The study is registered on the ClinicalTrials.gov public site; NCT01193933.
Methods and patients
From Jan 2009, till June 2014, 30 centers enrolled 250 pts: median age 30 years (15-60 years), 115f/135 m; in 2,4% phenotype was unknown (n=6), B-lineage ALL - 63,2% (n=158), T-lineage ALL - 34% (n=85), biphenotypic - 0,4% (n=1). Cytogenetics was available in 62,4% of patients (n=156) and 41% of them (n=64) had normal karyotype (NK). 25,2% of patients (n=63) were in the standard risk (SR) group (WBC <30 for B-lineage, <100 for T-lineage; EGIL BII-III, T-III; LDH < 2N; no late CR; t(4;11)-negative), 59,2% (n=148) - in the high risk (HR) group (WBC >30 for B-lineage, >100 for T-lineage; EGIL BI, T-I-II-IV; LDH > 2N; late CR; t(4;11)-positive), 39 patients were not qualified by risk group. The analysis was performed in June 2014. The median time of follow-up was 26,1 mo.
Results
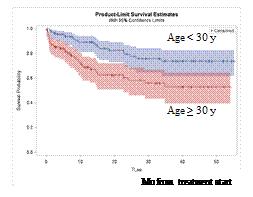
Prednisolone was replaced with dexamethazone after prephase in 68,1% of patients (135 of 198). The portion of non-responders to PRD in SR and HR groups was 54% and 67%, respectively (p=0,06). CR rate in 228 available for analysis patients was 87% (n=198), induction death occurred in 10,5% (n=24), resistance was registered in 2,5% (n=6). The majority of CR patients (91%) achieved it after prephase (7,1%, n=16) and the 1st phase of induction (83,9%, n=164). Late responders constituted 9% (n=18). None of those factors (PRD sensitivity, initial risk group, immunophenotype, late response) influenced overall (OS) or disease-free survival (DFS). OS rate in older ALL patients (>30) was substantially less than in younger ones (52,7% vs 73,6%, p=0,0009). DFS was comparable - 61,2% vs 71,5%, p=0,1. 24 of 75 (32%) CR T-ALL patients underwent autologous BMT after BEAM conditioning at a median time of 6 mo from CR and proceeded to further maintenance. No relapses were registered in this group so far. Allogeneic BMT was performed only in 14 patients (Ò-ALL-7, B-ALL-7) on the protocol.
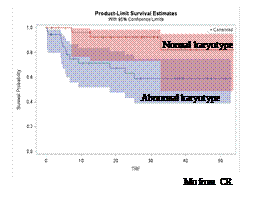
Totally 47 patients (20,6%) relapsed (16 with T-lineage, 31 with B-lineage ALL). At 48 mo OS for the whole group constituted - 65,6%, DFS - 69,3%. OS and DFS differed in B-ALL patients with NK in comparison with abnormal karyotype: 80% vs 57%,(p=0,01) and 81% vs 61%, respectively (p=0,02), but not in T-ALL patients.
Conclusions
Our data demonstrate that the proposed treatment approach is rather effective and reproducible. OS and DFS did not depend on various common risk factors (initial risk group, WBC, LDH, immunophenotype, late response, PRD resistance). The only independent risk factor for OS was age (>30 y). In B-cell ALL abnormal karyotype became an independent adverse risk factor for OS, DFS, relapse incidence.
Fig.1 Overall survival (A) according to age in the whole cohort and disease-free survival (B) in B-cell ALL according to karyotype
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal