Abstract
Background: Na/H exchanger 1 (NHE1), an important participant in the precise regulation system of intracellular pH (pHi), is known to be involved in pathological processes such as cell transformation, maintenance and active progression of the neoplastic process. Some studies have showed that leukemic cells showed higher pHi than normal cells, and NHE1 inhibitor could induce acidification and apoptosis of the leukemic cells. In this study, we tried to elucidate the role of NHE1 in leukemic cells according to cytarabine (AraC) resistance.
Materials and Methods: Two human AML cell lines, AraC sensitive (AS)-OCI-AML2 cells and AraC resistant (AR)-OCI-AML2 cells, primary leukemic cells from AML patients, and normal bone marrow mononuclear cells (BMMNC) from healthy donor were analyzed. The pH-sensitive fluorescent dye, 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) was used to measure pHi and NHE1 activity. The fluorescent ratio of the 490/440 nm was calibrated intracellularly. The expression of NHE1 was measured by qRT-PCR and western blot analysis. To inhibit the NHE1, the amiloride analogue, 5-(N,N-hexamethylene) amiloride (HMA) (10 µM, 20 µM, 30 µM) was used.
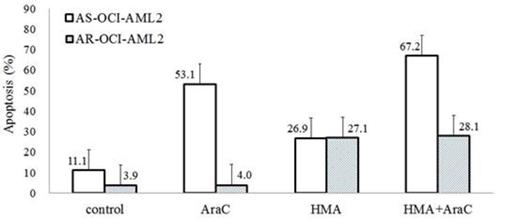
Results: To confirmed AraC sensitivity, cell lines were treated with 10 µM AraC for 24 hours, and apoptosis fraction in AS-OCI-AML2 cells and AR-OCI-AML2 cells were 53.1±7.2 % and 4.0±0.8 %, respectively. The pHi of AR-OCI-AML2 cells was significantly higher than AS-OCI-AML2 cells (7.839±0.033 vs. 7.589±0.129, P=0.045) and BMMNC (7.839±0.033 vs. 7.578±0.035, P=0.083), and these differences were associated with higher NHE1 activity. Compared AS-OCI-AML2 cells, AR-OCI-AML2 cells showed significantly higher NHE1 expression by western blot analysis (Figure 1), and NHE1 mRNA levels (0.039±0.014 vs. 1.565±0.070, P<.001) by qRT-PCR. Treatment with HMA (20 µM) could induce apoptosis both on AS-OCI-AML2 cells (26.9±2.8%) and AR-OCI-AML2 cells (37.4±18.8%). Interestingly, induction of apoptosis by HMA was dose-dependent both in AS-OCI-AML2 cells and AR-OCI-AML2 cells, and higher concentration of HMA (30 µM) could induce apoptosis on most of AR-OCI-AML2 cells (68.7±20.2%). Co-treatment experiment with 10 µM AraC and 20 µM HMA in AS-OCI-AML2 cells showed additive effect on inducing apoptosis (AraC vs. HMA vs. HMA+AraC = 53.1±12.4 vs. 53.1±12.4 vs. 67.20±4.3%, Figure 2), but in AR-OCI-AML2 cells, co-treatment did not show additional or synergistic effect on inducing apoptosis (AraC vs. HMA vs. HMA+AraC = 4.0±0.1 vs. 27.1±2.2 vs. 28.1±2.0%, Figure 2). As in the cell lines, primary leukemia cells from patients with AraC resistance showing higher pHi and NHE activity than those from patients without. HMA could induce apoptosis on primary cell lines regardless AraC sensitivity.
Conclusions: In this study, we first showed that NHE1 inhibition could induce apoptosis in leukemia cells regardless AraC sensitivity. Apoptotic activity was related with higher pHi and NHE activity in AraC resistant cell lines and primary leukemic cells. NHE inhibition induced apoptosis may be independent with AraC induced apoptosis. The heterogeneity in pHi and NHE activity within leukemic cells may be related to alteration in drug delivery machinery or dormant status of leukemia cells. Further experimental and clinical studies are needed to elucidate the therapeutic application of NHE1 inhibitor to AraC resistant AML.
Western blot analysis showed higher level of expression of Na/H exchanger I in AR-AML-OCI2 cells than AS-AML-OCI2 cells.
Western blot analysis showed higher level of expression of Na/H exchanger I in AR-AML-OCI2 cells than AS-AML-OCI2 cells.
Percentage of apoptotic cells after treatment with 20 µM HMA and/or 10 µM AraC.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal