Abstract
As immunomodulatory cytokines, Type 1 interferons (IFNs) have a long history of efficacy in treating chronic myeloid leukemia (CML). Recently, many research reported the combination of IFN-α and imatinib significantly increased the rates of molecular responses, comparing to single imatinib treatment. Related molecular mechanism may be the direct effect of IFN-alpha on stem cells. Therefore, IFN-α was renewed to be a vital candidate for CML treatment. Bone marrow mesenchymal stem cells (MSCs), which also be defined as mesenchymal stromal cells, are important to hematopoiesis. IFN-α was indicated as a potential inhibitor of MSCs; however the exact mechanism remains unclear. PML is known as a tumor suppressor, which locates at the downstream of IFN-α pathway. In our previous research, we have proved that PML stably expressed in human MSCs (hMSCs), which was important in maintaining the normal function of hMSCs. To our knowledge, although PML has been extensively studied in tumor cells, little is known about PML gene regulation in MSCs. In this study, we investigated the effect of IFN-α on hMSCs and the role of PML involved in this process.
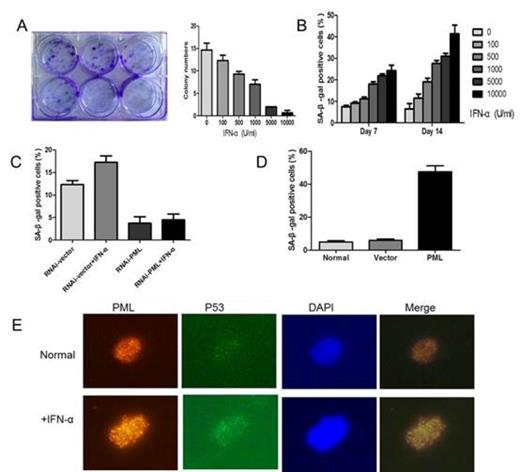
After approval by institutional review board, hMSCs were isolated from the bone marrow of volunteers and confirmed by flow cytometry. Cells were treated with different concentration of IFN-α up to 14 days. We found that IFN-α treated cells were growing slowly, and had a dramatically decreased number of colone in a dose dependent manner (Fig A). However, IFN-α did not induce significant cell apoptosis. Then a variety of senescence-associated detection was measured. hMSCs senescence induced by IFN-α had a dose and time dependent manner (Fig B). After treated with IFN-α at 1000 U/ml for 7 or 14 days, we found that up to 18% ± 1.1 or 27.56% ± 1.33 of hMSCs became SA-b-gal-positive as compared with 7.53% ± 0.55 or 6.47% ± 2.5 of untreated cells (P<0.05 for both). Real time PCR analysis proved this process by an increase in production of the senescence marker p53 and p21.
Expression of PML was detected by real-time PCR and immunofluorescence in hMSCs treated with IFN-α. Consistent with other studies, mRNA expression of PML can be up-regulated by IFN-α in hMSCs. When cells were treated with IFN-α at 1000 U/ml for 7 or 14 days, PML gene expression in hMSCs was increased by more than 2 fold. At the same time, both the number and size of PML-NBs were increased markedly and had a concentration dependent manner. These results indicate that PML protein can be up regulated by IFN-α in hMSCs.
Then, PML expression was inhibited using an RNAi-mediated PML knockdown system. After treated with IFN-α at 1000 U/ml for 7 days, hMSCs senescence can be rescued by the knocking down of PML. The percentage of SA-b-gal positive cells in PML knocking down hMSCs has a significant decrease as compared with cell transfected with control-RNAi (4.49% ±1.27 vs. 17.26% ± 1.44, P < 0.05) (Fig C). To further characterize the effect of PML on cellular senescence in hMSCs, PML-overexpressed hMSCs were used. 7 days post-transfection, PML overexpressing hMSCs were strongly positive for SA-b-gal activity (47.43%±3.8), as compared with normal and empty vector transfected cells (4.9%±0.7, 5.97%±0.75) (P< 0.001) (Fig D). mRNA levels of P53 and P21 were also enhanced in PML-overexpressed hMSCs.
P53 pathway contributes to cell senescence and the role of PML has been proved in the regulation of P53 activity, we wondered whether upregulation of PML induced by IFN-α has relationship with P53 pathway in hMSCs. In the process of IFN-α induced hMSCs senescence, an increasing co-localization of PML and P53 was observed in IFN-α treated cells (1000U/ml, 7 days) as compared with untreated cells (Fig E). To further confirm whether or not the change of P53 location was mediated through the upregulation of PML, we knocked-down the expression of PML in hMSCs. Treated with IFN-α (1000U/ml, 7 days), we did not found significant location of P53 in PML-knocking down cells as compared with control.
Taken together, our results suggested that hMSCs incurred senescence upon IFN-α stimulation, while PML levels were observed significant increase. By knocking-down and overexpressing PML, we demonstrated that PML was indispensable to IFN-α mediated hMSCs senescence. The molecular mechanisms underlying this process may be an increased co-localization of PML and p53 induced by IFN-α. These findings provided a novel insight into the role of IFN-α on hMSCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal