Abstract
Background. The combination of melphalan and dexamethasone (MDex) is considered standard treatment for patients with AL amyloidosis who are not eligible for autologous stem cell transplant at most referral centers. When full-dose (40 mg) dexamethasone can be combined with melphalan, hematologic response is achieved in three fourths of patients, with complete remissions (CRs) in 30% of cases and prolonged survival (median >7 years). Several studies showed that bortezomib is highly effective in AL amyloidosis with response rates as high as 80-90%, with 50-60% CRs, when used in combinations with alkylating agents and dexamethasone, suggesting that these regimens could replace current standard of care in AL amyloidosis. A recent case-control study indicated that the addition of bortezomib to MDex (BMDex) does not overcome the poor prognosis of patients with advanced cardiac involvement. Here we report the first interim analysis of a multicenter randomized phase III trial comparing MDex and MDex with the addition of bortezomib (BMDex) in newly-diagnosed AL amyloidosis ongoing in Europe and Australia (EMN-03 study, NCT01277016).
Patients and Methods. Main eligibility criteria included measurable disease (M-protein >10 g/L or dFLC >50 mg/L), estimated glomerular filtration rate (eGFR) ³30 mL/min, and adequate liver function. Previously treated patients, those who had >30% bone marrow plasma cell or lytic bone lesions, NYHA class >II heart failure, grade 3 sensory or grade 1 painful peripheral neuropathy, or ECOG performance status >2 were excluded. In January 2013 the protocol was amended to include Mayo stage III patients, provided their NT-proBNP was <8500 ng/L. Patients were randomized to receive either MDex (melphalan at 0.22 mg/kg and dexamethasone at 40 mg daily for 4 consecutive days every 28 days) or BMDex (bortezomib added at 1.3 mg/m2, on days 1, 4, 8, and 11 in cycles 1 and 2, and on days 1, 8, 15, and 22 in the following cycles). Treatment was continued until completion of MDex cycle 9 or BMDex cycle 8, or achievement of CR or of at least partial response (PR) plus organ response after cycle 6, and was discontinued in case PR was not achieved by cycle 3. Planned enrollment was 110 patients; since January 2011, 70 subjects have been enrolled, 35 in each arm (database lock: July 25, 2014).
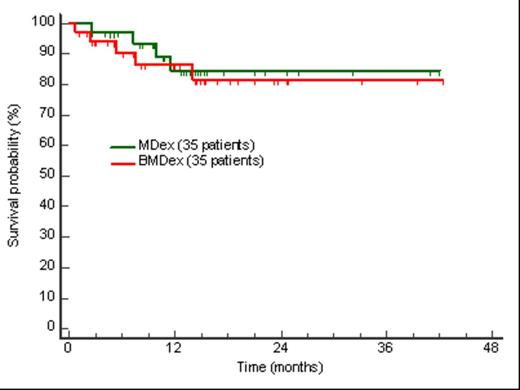
Results. Heart and renal involvement were present in 26 (74%) and 22 (63%) patients in the MDex arm, and in 25 (71%) and 24 (69%) in the BMDex arm, respectively. Five patients in each arm were stage III. In the MDex arm 15 patients (43%) experienced grade 3-4 adverse events [cytopenia (6), fluid retention (4), fatigue (2), need for a coronary stent (1), diarrhea (1), and renal failure (1)]. In the BMDex arm grade 3-4 adverse events were reported in 21 patients (63%, P=0.151 compared to MDex): cytopenia (12, febrile in 1 case), fluid retention (3), insomnia (2), transient troponin increase (1), peripheral neuropathy (1), renal failure (1), and injection site reaction (1). Three patients died in the first three months, 1 was treated with MDex and 2 with BMDex. Response was evaluated by intent to treat. Fifty-one patients, 26 treated with MDex and 25 who received BMDex, completed cycle 3 or died before completion and are evaluable for response. Overall, 15 patients (58%) responded in the MDex arm, and 19 (76%) in the BMDex arm (P=0.166). After cycle 3, nine patients (35%) in the MDex group and 16 (65%) in the BMDex group achieved at least very good partial response (P=0.036). Renal response was achieved in 2 of 8 evaluable patients in the MDex group and in 2 out of 10 subjects in the BMDex arm. Cardiac response was reached in 4 of 15 evaluable patients in the MDex arm and in 2 out of 12 in the BMDex arm. After a median follow-up of 14 months, 9 patients (13%) died, 4 in the MDex arm and 5 in the BMDex arm (Figure 1).
Conclusion. This is the first prospective randomized trial of novel agents in AL amyloidosis. The present interim analysis indicates that the addition of bortezomib to MDex grants more profound hematologic responses that should be balanced with relative increase in toxicity. Longer follow-up is required to demonstrate a benefit in terms of organ improvement and of overall survival. Updated data will be presented at the meeting.
We would like to acknowledge the European Myeloma Network and the Leukaemia Foundation of Australia for their ongoing support, and Janssen-Cilag for partially funding the trial and providing the study drug.
Patients’ survival
Leleu:Janssen, Celgene, leopharma, Takeda, Amgen, Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Zamagni:Janssen and Celgene: Honoraria. Cibeira:Janssen and Celgene: Honoraria. Schönland:Janssen: Honoraria. Moreau:Millenium and Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Hajek:Merck and Celgene: Consultancy, Honoraria; Janssen: Honoraria. Mateos:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dimopoulos:Celgene and Orthobiotech: Honoraria. Sonneveld:Celgene, Janssen, Onyx, Millennium: Research Funding; Celgene, Janssen, Onyx, Millennium: Membership on an entity's Board of Directors or advisory committees. Merlini:Millennium Takeda: Honoraria. Off Label Use: Bortezomib in AL amyloidosis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal