Abstract
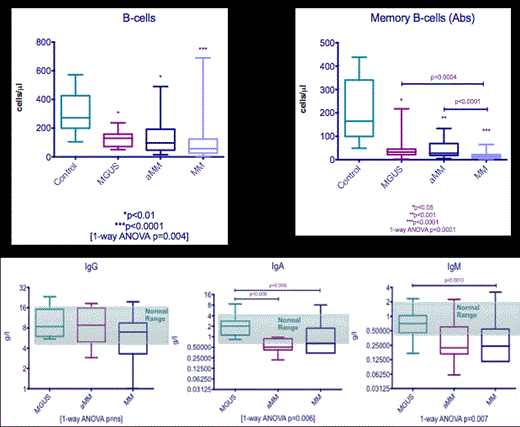
Multiple myeloma (MM) is characterized by an increased risk of infection due to the immunosuppressive effect of the disease and conjointly of therapy. Furthermore, there is impaired responses to vaccination to counter the infection risk. The factors that underpin defective B-cell homeostasis and effective humoral immunity are not clear, nor are the extent of the defects. Also, the level of impaired humoral immunity in MGUS is not fully understood. The aim of this study was to delineate the circulating B-cell populations and recall antibody responses in patients with MGUS & MM, compared to age-matched controls, correlating with the responsiveness to vaccinations, incidence of infective complications and concomitant therapy. We performed comprehensive B-cell immunophenotyping by multi-parameter flow cytometry of peripheral blood samples from patients with MGUS (n=16), asymptomatic MM (n=18) and MM (n=108) with a median age of 63 years (range 38-94) comparing them to age-matched controls (n=9). B-cell subsets included naïve (CD19+CD27-), memory (CD19+CD27+; non-switch CD19+IgD+CD27+, switch CD19+IgD-CD27+), transitional (CD19+CD27-CD24hiCD38hi) & regulatory (CD19+CD27+CD24hi) B-cells. Serum uninvolved total IgG, IgM & IgA levels along with vaccine-specific antibody responses were analysed. There is a progressive decrease in the uninvolved immunoglobulin classes with significant reduction in total IgA (p=0.006) and IgM levels (p=0.007) in aMM/MM compared to MGUS & control (Figure 1). When anti-pneumococcal antibodies were measured, only 30% of aMM/MM patients had adequate protective levels compared to 79% of age-matched controls, with 40% of aMM/MM patients with inadequate levels experiencing recurrent respiratory tract infections compared to 25% of aMM/MM patients with adequate proactive antibodies. Patients with MGUS, aMM and MM have lower total B-cell numbers compared to controls (1-way ANOVA p=0.004; Figure 1). The reduction in B-cell numbers were primarily the consequence of reduced memory B-cells (percentage and absolute 1-way ANOVA p<0.0001), noted in both MGUS and aMM/MM but a progressive reduction with increasing disease activity (MGUS>aMM>MM). Furthermore, a correlation with total IgG levels & memory B-cell numbers is evident (r2=-0.053) & progressive reduction in memory B-cell numbers is seen with advancing cycles of therapy. The ratio of switch:non-switch memory B-cells is unaltered (control 1.05, MGUS 0.53, aMM 1.41 & MM 1.49; 1-way ANOVA p=ns). Conversely, there is a compensatory increase in the percentage of transitional B-cells when increasing disease stage is compared to controls (control 7.38% (95%ci 4.9,9.9) vs MGUS 14.0% (95%ci 7.4, 20.7) vs aMM 14.95% (95%ci 8, 21.9); 1-way ANOVA p<0.001) but a reduction is noted in MM (5.82%, 95%ci 4.5,7.2; p<0.0001), primarily being driven by sequential lines of therapy. As a consequence, the ratio of Memory:transitional B-cells is significantly reduced in aMM/MM compared to MGUS & controls (control 10.35, MGUS 20.46, aMM 7.74 & MM 4.57; 1-way ANOVA p=0.006), associated with increasing incidence of bacterial infections. A non-significant correlation is seen between transitional B-cells and total uninvolved immunoglobulin levels and with recall responses to vaccinations. There is a progressive decrease in the CD19+CD27+CD24hi B-cell subset between control and plasma cell dyscrasias (control 20.4% (95%ci 15.5,25.2), MGUS 14.0% (95%ci 7.4, 20.7), aMM 14.95% (95%ci 8, 21.9) & MM 5.82%, 95%ci 4.5,7.2; p<0.0001), primarily being driven by sequential lines of therapy and associated with increased incidence of infection. This study illustrates that patients with myeloma demonstrate reduced total circulating B-cells primarily as a consequence of reduced memory B-cells, associated with reduced immunoglobulin and recall antibody responses. This is associated with increased incidence of bacterial infections and is worsened by sequential exposure to lymphodepleting therapies. Of particular importance is the identified aberration in B-cell subsets seen in MGUS compared with age-matched control, indicative of humoral immune dysregulation highlighting that MGUS may not be an immunologically inert disorder.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal