Abstract
Background: Clinical trials report that chemoimmunotherapy with rituximab (R) improves overall survival (OS) and progression-free survival in the treatment (tx) of symptomatic CLL patients (pts). R has been available for first-line CLL tx in BC, population 4.5 million, since 2004. We compared clinical outcomes with and without addition of R to chemotherapy in a large unselected provincial cohort of pts treated for CLL, to determine the "real world" effectiveness of addition of R to standard chemotherapy.
Methods: Three large provincial databases (db) were used to identify eligible pts: the BC Provincial CLL db, the BC Lymphoid Cancer db, and the Providence Hematology CLL db. All pts who received minimum 1 cycle of first-line tx for confirmed CLL were included. Pts with > 1 hematologic malignancy (n=8) were excluded. Baseline features of pts treated with (+R) or without R (No R) were compared using Chi-squared for categorical and Kruskal-Wallis test for continuous variables. OS was calculated from date of initial tx to date of death from any cause. Treatment-free survival (TFS) was calculated from date of initial tx to date of next tx/death from any cause. Multivariate analysis (MVA) was performed using Cox proportional hazard models to evaluate the effect of R on OS/TFS, after controlling for covariates including age (³60 yrs vs <60 yrs), Rai stage (3-4 vs 0-2), CD38 status (pos vs neg), presence of 17p (17p-) and 11q (11q-) deletions, and first-line tx with purine analogs (PAs).
Results: A total of 1784 pts diagnosed with CLL from 1973-2014 were identified, of which 726 pts (41%) received tx in follow-up. Of treated pts, 393 (54%) received R and 333 (46%) received chemotherapy alone. Among the No R group, tx included: chlorambucil 56%; fludarabine (F) 34%; cyclophosphamide (C)-based 8%; cladrabine 2%. Among the +R group, tx included: FR 75%; C-based + R 17%; FCR 7%; chlorambucil + R 1%. 103 pts underwent bone marrow transplant (BMT) during their tx course (19% No R vs 10% +R, P=.002). Median age at diagnosis (dx) and tx between groups were not statistically different (No R vs +R: 60.6 vs 60.8 yrs and 64.7 vs 63.9 yrs, respectively). There were no clinically significant differences in diagnostic parameters including % with elevated LDH, lymphocyte count >20x109/L , Rai stage 3-4. Median follow-up time in survivors was longer in the No R group (13.0 vs 6.8 yrs, P<.001). Among 467 pts with known CD38 status, CD38 pos was more common in +R vs No R groups (47 vs 36%, P=.02). FISH was performed in 586 pts, with no significant differences in abnormalities between tx groups. Poor-risk FISH, 17p- or 11q-, were present in 29% (No R) and 27% (+R).
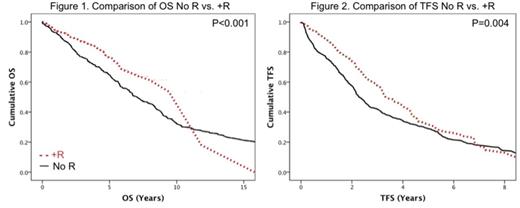
Median time from dx to initial tx was 2.8 yrs (range 0-20.6) in No R vs 2.5 yrs (range 0-22.7) in +R groups (P=.84). OS was longer in the +R cohort (median OS 11.8 vs 7.1 yrs, P<.001), Fig. 1. Significant improvements in OS were also seen in pts <60 yrs of age at tx (median OS 11.3 vs 3.1 yrs, P<.0001), without 17p- (median OS 9.3 vs 5.2 yrs, P<.0001), and treated with PAs (median OS 9.4 vs 6.4 yrs, P=.0001). Median TFS was longer in pts treated with R (3.3 vs 2.3 yrs, P= .004), Fig. 2, and in those without 17p- (median TFS 3.1 vs 1.3 yrs, P<.001).
MVA confirmed that the addition of R to chemotherapy remained a strong independent predictor of mortality (HR 0.66, 95% CI: 0.44-0.98, P=.04) and TFS (HR 0.6, 95% CI: 0.46-0.79, P<.001) after controlling for covariates. Other independent predictors of OS included age ³60 yrs (HR 2.77, 95% CI 1.87-4.10, P<.001) and presence of 17p- (HR 1.23, 95% CI 1.62-3.76, P<.001), whereas for TFS, presence of 17p- (HR 2.08, 95% CI: 1.49-2.91, P<.001) and CD38+ (HR 1.32, 95% CI: 1.03-1.68, P=.025) were independent negative predictors.
Conclusion: In this large, population based cohort of pts treated for CLL, we confirm that the addition of R to chemoimmunotherapy as initial tx significantly improves OS, resulting in a 44% lower risk of overall mortality (95% CI, 2% to 66%) after controlling for covariates. We have also demonstrated that the addition of R to first-line therapy significantly delays the time to subsequent therapy, a finding not previously reported in a population based setting to our knowledge. This study complements clinical trial [Hallek, Lancet 2010] and US Registry data [Danese, Blood 2011], demonstrating benefit of the addition of R to standard therapy for first-line treatment of CLL and shows the generalizability of such results in a real world setting.
Gerrie:Roche: Honoraria, Research Funding. Ramadan:Roche: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal