Abstract
BACKGROUND: PI3Kδ signaling is critical for the proliferation and survival as well as for homing and tissue retention of malignant B cells. Idelalisib is a first-in-class, targeted, highly selective, oral inhibitor of PI3Kδ with considerable activity as monotherapy and in combination with R in patients with relapsed/refractory CLL, and was recently approved for the treatment of relapsed CLL in combination with R. This report summarizes the long-term follow-up of the Phase 1 combination experience of idelalisib with chemo- and immuno-chemotherapies.
METHODS: 74 subjects were sequentially enrolled into one of 5 regimens of idelalisib combined with either B (70 or 90 mg/m2 IV on D1,2 of cycles 1-6, N=18, enrolled Apr 2011-Nov 2011), BR (B: 70 mg/m2 IV on D1,2 of cycles 1-6; R: 375 mg/m2 IV on D1 of cycles 1-6, N=15, enrolled Apr 2011-Aug 2011), F (40 mg/m2 po D1-5 of cycles 1-6, N=12, enrolled Apr 2011-Aug 2011), Chl (10 mg/m2 po D1-7 x 3(min)-12(max) cycles, N=15, enrolled Mar 2012-Aug 2012), or ChlR (N=14, enrolled Mar 2012-Jul 2012). Idelalisib was administered at 100 (4 of the pts receiving B) or 150 mg po BID (all other patients) continuously.
Patients on treatment after 48 weeks were eligible to continue idelalisib on an extension study. Clinical responses were evaluated according to published criteria (Hallek 2008; Cheson 2012).
RESULTS: Of 74 subjects enrolled, 66% were male. Median age was 65 years, and median time since diagnosis was 8 years with a median of 3 (range 1-9) prior regimens. Overall, prior therapies included R (97%), F (78%), B (45%), and Chl (14%), and 55% of patients were refractory to last therapy. 65% of patients had Rai stage III/IV disease at enrollment. Adverse prognostic factors included del(17p) and/or TP53 mutation (30%), IGHV unmutated status (82%), NOTCH1 mutation (28%).
As of 7/15/2014, the median idelalisib exposure for patients of all cohorts was 12.8 (range 1-48) months. 41 (55%) of patients completed the 48 weeks of the primary study, and 20 subjects (27%) were still on treatment on the extension study at the time of analysis.
The most common reasons for discontinuation reported by investigators were adverse events (AEs) (15, 20%) or progressive disease (PD) (12, 16%). There were 13 deaths reported on study; 4 patients experienced PD before death.
Selected treatment-emergent AEs (any Grade/≥Gr 3, regardless of causality) included diarrhea/colitis (51%/18%), pyrexia (47%/5%), fatigue (35%/7%), cough (35%/0%), nausea (31%/1%), pneumonia (23%/14%), rash (22%/7%), dyspnea (19%/3%), and pneumonitis (3%/3%). Elevation of liver transaminases (TA, any Grade/≥Gr 3) was seen in 37%/14%. Of those, only 1 patient discontinued the study because of (recurrent) TA elevation. Richter’s transformation as category of progressive disease was reported in 1 patient (3%).
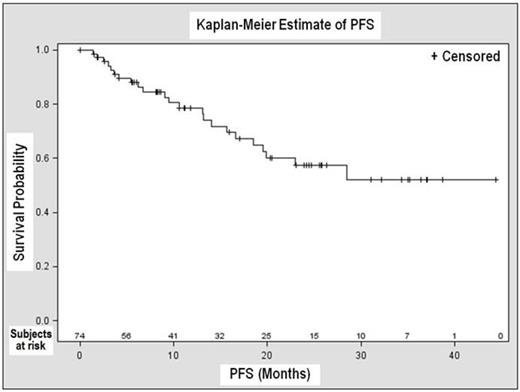
The ORR was 82% for all 74 patients with a CR rate of 10%. 4 of 74 patients (5%) were not evaluable because of missing follow-up assessments. Among 69 subjects with genetic data available, the ORR in the 22 subjects with either del(17p) and/or TP53 mutation was 68% vs. 87% among 47 subjects with neither aberration. The overall median PFS was not reached at the time of analysis (Figure 1); KM estimate of PFS was 57% [43-72%, 95% CI] at 24 months. Overall median time to response was 1.9 months, and overall median DOR was not yet reached.
CONCLUSIONS: These preliminary results in patients with relapsed or refractory disease and multiple prior therapies suggest that idelalisib can be combined with B, BR, F, Chl, and ChlR with acceptable safety and that these combinations have substantial clinical activity. A Phase 3 study evaluating the combination of idelalisib with BR in relapsed CLL is ongoing (NCT01732926).
Overall PFS
Barrientos:Gilead Sciences: Research Funding. Off Label Use: Zydelig is a kinase inhibitor indicated for the treatment of patients with: 1) Relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients for whom rituximab alone would be considered appropriate therapy due to other co-morbidities; 2) Relapsed follicular B-cell non-Hodgkin lymphoma (FL) in patients who have received at least two prior systemic therapies; and 3) Relapsed small lymphocytic lymphoma (SLL) in patients who have received at least two prior systemic therapies.. Coutre:Gilead Sciences: Research Funding. de Vos:Gilead Sciences: Research Funding. Wagner-Johnston:Gilead Sciences: Research Funding. Flinn:Gilead Sciences: Research Funding. Sharman:Gilead Sciences: Research Funding. Schreeder:Gilead Sciences: Research Funding. Boyd:Gilead Sciences: Research Funding. Rai:Gilead Sciences: Research Funding. Leonard:Gilead Sciences: Research Funding. Kim:Gilead Sciences: Employment, Equity Ownership. Viggiano:Gilead Sciences: Employment, Equity Ownership. Jahn:Gilead Sciences: Employment, Equity Ownership. Furman:Gilead Sciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal