Abstract
Introduction:
ABT-199 is a selective, orally bioavailable BCL-2 inhibitor that rapidly induces responses in ~80% of patients (pts) with relapsed/refractory (R/R) CLL or small lymphocytic lymphoma (SLL) as a single agent (Seymour, EHA 2014). Rituximab (R) has only modest and short-lived activity as a single-agent in CLL; it can enhance the activity of other agents with minimal additional toxicity. ABT-199 and R demonstrate synergy in preclinical models of CD20-positive lymphoid malignancies, and the combination has the potential to improve efficacy.
Methods:
Primary objectives of this phase 1b, open-label, dose-escalation, multicenter study were to determine the maximum tolerated dose (MTD)/recommended phase 2 dose (RPTD) of ABT-199 + R in pts with R/R CLL/SLL and evaluate the safety profile; secondary objectives examine the pharmacokinetics (PK) and efficacy of the combination. Eligible pts began treatment with 20 or 50 mg ABT-199 (modified to 20 mg during the study) daily, with weekly increases in a ramp-up phase to a final cohort dose of 200 - 600 mg. After completion of the ramp-up phase, R was added starting at 375 mg/m2 and then 500 mg/m2 monthly for a total of 6 doses. ABT-199 was continued daily until progressive disease (PD) or unacceptable toxicity. Adverse events (AEs) were graded according to NCI-CTCAE v4.0. The MTD was determined using the Continual Reassessment Method. Responses were assessed using established criteria for CLL/SLL, including CT scan at month 3 and CT scan and bone marrow (BM) biopsy at the end of combination therapy. MRD was assessed using four color flow cytometry in peripheral blood (PB) and/or BM aspirate ≥2 months after response criteria were first met, and in PB every 12 weeks until MRD negativity was achieved.
Results:
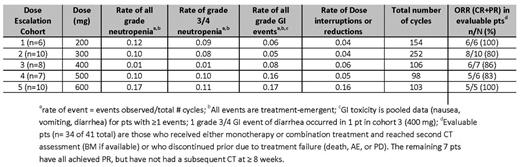
As of July 6, 2014, 49 pts enrolled in 5 dose escalation cohorts (n = 41) and a safety expansion cohort (n = 8), with a median time on study of 201 days (1 – 624). Median age was 68 years (range 50–88) and 30 (61%) were male. The median number of prior therapies was 3 (1–10). Thirteen (27%) had fludarabine-refractory disease and 9 (18%) R-refractory. Nine (18%) had del (17p) and 19/27 (70%) with available data had unmutated IGVH. Thirteen pts have discontinued therapy: 6 due to PD; 3 after attaining complete remission; 2 due to AEs and 2 withdrew consent. Preliminary PK data suggest a negligible effect of R on ABT-199 exposure. The most common overall treatment-emergent AEs (>25% pts) were neutropenia (47%), nausea (41%), diarrhea (37%), pyrexia (31%), headache (31%), fatigue, upper respiratory tract infection, and cough (each 29%). The most common overall grade 3/4 AEs were neutropenia (47%), thrombocytopenia (16%), and anemia (14%). Treatment-emergent SAEs occurred in 20 (41%) pts; the most common were pyrexia (6%), and febrile neutropenia, infusion-related reaction, TLS, and Richter's Transformation (each 4%). Six pts experienced a DLT: 1 febrile neutropenia (200mg), 1 each thrombocytopenia, hematophagocytic syndrome, neutropenia (300mg), 1 neutropenia (600mg), and 1 pt experienced a fatal event of hyperkalemia in the setting of TLS after the first dose (50mg). That event led to a modified dosing scheme; no TLS was observed in the 32 subsequent pts using the revised ramp up dosing scheme. No MTD was identified. Of 34 pts who were evaluable for response in dose escalation cohorts, the overall response rate (ORR) was 88%, with 11 (32%) achieving a CR/CRi and 20 (56%) achieving a partial response (PR). MRD was quantified by local laboratory in 19 pts. Thirteen pts (7 in CR (6 at 10-4 and 1 at 10-3 sensitivity) and 6 in PR (10-4 sensitivity)) were MRD negative in bone marrow while one was MRD negative in peripheral blood (10-4 sensitivity). The RPTD of ABT-199 is 400 mg daily based on safety data; key safety and efficacy data for the dose selection are summarized in the table.
Conclusions:
The combination of ABT-199 and R is well-tolerated and achieves an overall response rate of 86% with 31% CRs in pts with CLL/SLL. Efficacy was observed across all dose cohorts. The RPTD of ABT-199 is 400mg daily, supported by a trend of lower rates of neutropenia and GI toxicity events in the absence of a clear difference in efficacy, compared to the higher doses. A phase 3 trial comparing ABT-199 and R versus bendamustine and R in pts with previously treated CLL is underway.
Roberts:Walter and Eliza Hall Institute of Medical Research : Employment, Research Funding; AbbVie: Research Funding; Genentech: Research Funding. Ma:Genentech: Consultancy; AbbVie: Research Funding. Kipps:AbbVie: Consultancy, Research Funding. Barrientos:Gilead: institutional funding Other; Pharmacyclics: Consultancy, institutional funding, institutional funding Other; AbbVie: institutional funding, institutional funding Other; Celgene: Consultancy. Davids:Genentech: Consultancy; Infinity Pharmaceuticals: Consultancy. Anderson:Walter and Eliza Hall Institute of Medical Research: Employment, milestone payments related to ABT-199 Other; AbbVie: Research Funding; Genentech: Research Funding. Tam:Roche: Honoraria. Mason-Bright:AbbVie : Employment. Rudersdorf:AbbVie: Employment. Gressick:AbbVie: Employment. Yang:AbbVie, Inc: Employment. Munasinghe:AbbVie: Employment. Zhu:AbbVie, Inc: Employment. Cerri:AbbVie: Employment. Enschede:AbbVie, Inc: Employment. Humerickhouse:AbbVie, Inc: Employment. Seymour:AbbVie: Research Funding; Genentech: Consultancy, Research Funding; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal