Abstract
Juvenile Myelomonocytic Leukemia is the most common pediatric myeloproliferative neoplasm (MPN). JMML is characterized by myeloid populations with mutually-exclusive mutations in Ras-Erk signaling genes, most commonly PTPN11, which confer growth hypersensitivity to GM-CSF. JMML is notable among pediatric MPNs as being refractory to chemotherapy and having a 50% relapse rate following allogeneic hematopoietic stem cell (HSC) transplantation. As such, there is an urgent need for novel JMML therapies. The recent discovery of yolk sac myeloid lineages that persist into adulthood independently of bone marrow HSC contributions suggests a mechanism for JMML relapse following HSC transplantation. In this study, we sought to determine whether yolk sac HSC-independent myeloid progenitors bear hallmarks of MPN in a mouse model of JMML.
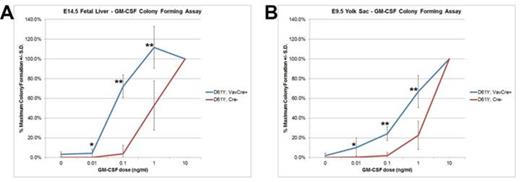
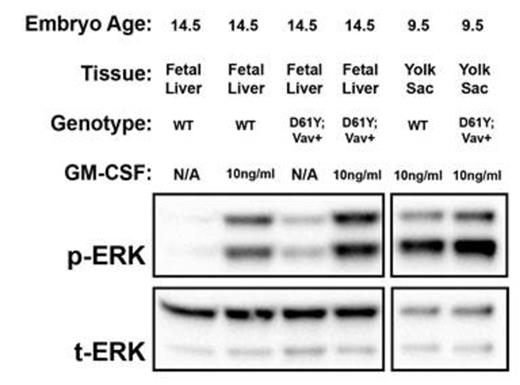
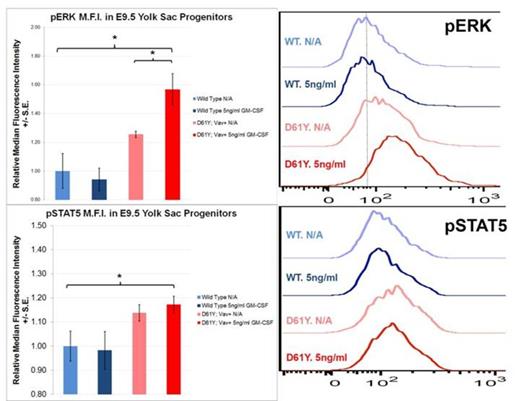
Using the Vav1 promoter-directed Cre recombinase, we generated a mouse model of JMML that expresses the PTPN11D61Y gain of function mutation in all waves of embryonic and adult hematopoiesis, including yolk sac myeloid progenitors that emerge prior to and independently from HSCs. PTPN11D61Y/+; VavCre+ mice are viable, born at expected Mendelian ratios, and develop peripheral blood monocytosis as early as 4 weeks of age. Given this early onset, we hypothesized MPN may develop in these mice during embryonic development. E14.5 fetal liver progenitors from PTPN11D61Y/+; VavCre+ embryos displayed marked GM-CSF hypersensitivity in methylcellulose colony forming assays (Figure-1A), possessed hyperactive Ras-Erk pathway signaling (Figure-2), and had a skewed progenitor distribution with a greater proportion of megakaryocyte-erythroid progenitors (63.5% vs. 50.1%, p<0.01) and fewer common myeloid progenitors (9.2% vs. 19.3%, p<0.01) than littermate controls. Since the E14.5 fetal liver contains both HSC-dependent and HSC-independent myeloid progenitors, we repeated these experiments using E9.5 yolk sac samples to restrict our analysis to HSC-independent hematopoiesis. Compared to littermate controls, PTPN11D61Y/+; VavCre+ E9.5 embryos had no difference in overall number of yolk sac myeloid progenitors (Ter119-; cKit+, CD41Dim), and in the number and distribution of CFU-E/GM/GEMM colonies in methylcellulose assay. However, PTPN11D61Y/+; VavCre+ yolk sac progenitors demonstrated marked GM-CSF hypersensitivity in colony forming assay (Figure-1B) as well as hyperactive Ras-Erk signalling by western blot analysis (Figure-2) and intracellular flow cytometry with antibodies against pSTAT5 and pERK (Figure-3).
We have demonstrated that HSC-independent myeloid lineages from the murine yolk sac possess GM-CSF hypersensitivity and Ras-Erk pathway hyperactivation in a mouse model of JMML. These findings suggest that HSC-independent hematopoietic populations may be involved in the development of JMML. Our study highlights the need to further assess the role of bone marrow-independent myeloid lineages in pediatric MPN, and to identify innovative therapies that can specifically target HSC-independent hematopoietic lineages.
Embryonic myeloid progenitors from PTPN11D61Y/+; VavCre+ embryos demonstrate GM-CSF growth hypersensitivity. A) E14.5 fetal liver mononuclear cells (n=7) or B) E9.5 yolk sac cells (n=8) were plated in methylcellulose colony forming assays and colonies were counted 7 days later. * p<0.05; ** p<0.001 by two-tailed Student’s t-test.
Embryonic myeloid progenitors from PTPN11D61Y/+; VavCre+ embryos demonstrate GM-CSF growth hypersensitivity. A) E14.5 fetal liver mononuclear cells (n=7) or B) E9.5 yolk sac cells (n=8) were plated in methylcellulose colony forming assays and colonies were counted 7 days later. * p<0.05; ** p<0.001 by two-tailed Student’s t-test.
Western blot analysis demonstrates hyperactive Ras-Erk signaling in E14.5 fetal liver and E9.5 yolk sac progenitors from PTPN11D61Y/+; VavCre+ embryos. Cultured progenitors were starved overnight and stimulated with GM-CSF for 60min prior to protein extraction. p, phosphorylated protein; t, total protein; WT, wild type.
Western blot analysis demonstrates hyperactive Ras-Erk signaling in E14.5 fetal liver and E9.5 yolk sac progenitors from PTPN11D61Y/+; VavCre+ embryos. Cultured progenitors were starved overnight and stimulated with GM-CSF for 60min prior to protein extraction. p, phosphorylated protein; t, total protein; WT, wild type.
Yolk Sac Myeloid Progenitors from PTPN11D61Y/+; VavCre+ embryos demonstrate Ras-Erk pathway hypersensitivity at baseline and following GM-CSF stimulation. Cultured yolk sac progenitors were stimulated for 30min with 5ng/ml of GM-CSF, processed for intracellular flow cytometry, and stained with the indicated fluorescent antibody. Histograms display representative median fluorescence intensity among CD45+ cells in each sample (n=3). * p<0.05 by two-tailed Student’s t-test. ADDIN EN.REFLIST
Yolk Sac Myeloid Progenitors from PTPN11D61Y/+; VavCre+ embryos demonstrate Ras-Erk pathway hypersensitivity at baseline and following GM-CSF stimulation. Cultured yolk sac progenitors were stimulated for 30min with 5ng/ml of GM-CSF, processed for intracellular flow cytometry, and stained with the indicated fluorescent antibody. Histograms display representative median fluorescence intensity among CD45+ cells in each sample (n=3). * p<0.05 by two-tailed Student’s t-test. ADDIN EN.REFLIST
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal