Abstract
Background: In 2 phase III studies comparing the Janus kinase (JAK) 1 and JAK2 inhibitor ruxolitinib with placebo (Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment I [COMFORT-I]) or best available therapy (COMFORT-II), ruxolitinib treatment was associated with rapid and durable reductions in spleen volume, improved myelofibrosis (MF)-related symptoms and quality of life measures, and prolonged survival. However, the key measures of efficacy used in the COMFORT studies—magnetic resonance imaging/computed tomography (MRI/CT) to measure change in spleen volume and the modified Myelofibrosis Symptom Assessment Form v2.0 to assess change in MF-related symptoms—are not commonly used in clinical practice. Therefore, we analyzed the efficacy of ruxolitinib in COMFORT-I using practical, community-based measures of clinical benefit (spleen palpation and the Patient Global Impression of Change [PGIC] questionnaire).
Methods: In COMFORT-I, patients (N = 309) with primary MF, post–polycythemia vera MF or post–essential thrombocythemia MF were randomized to receive ruxolitinib (n = 155) or placebo (n = 154). For this analysis, patients were divided into groups by degree of palpable spleen reduction and symptom improvement at week 12 as follows: patients with clinically relevant reductions in palpable spleen as defined by the International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus criteria (SPL), patients with “much improved” or “very much improved” symptoms as measured by the PGIC (PGIC), or patients who did not achieve SPL or PGIC (Neither). The Neither group encompassed patients with “minimally improved” symptoms with no worsening in palpable spleen or patients who achieved 10% reduction in palpable spleen with no worsening in symptoms (Minor), and patients who did not achieve any palpable spleen reduction or PGIC-assessed symptom improvement (None). Nonevaluable patients (4/155 for ruxolitinib and 13/154 for placebo) were not included in the analysis. Additional measures of clinical benefit assessed at week 12 included changes in spleen volume and changes in white blood cell (WBC) count. Durability of all measures of clinical benefit with longer-term ruxolitinib therapy was also evaluated.
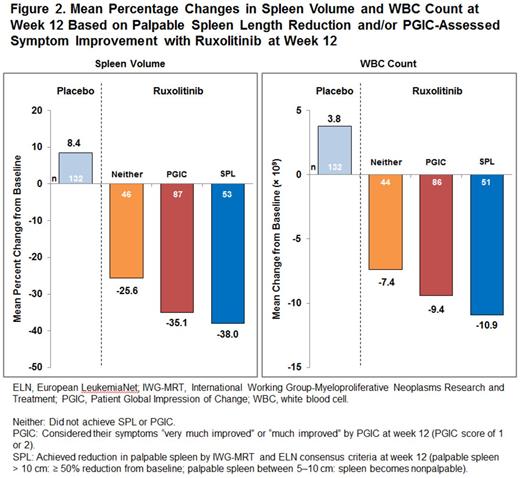
Results: At week 12, the majority (91.4%) of patients randomized to ruxolitinib achieved some clinical benefit as measured by palpable spleen reduction and/or PGIC-assessed symptom improvement, vs 31.9% of those assigned to placebo (Figure 1). Benefits in palpable spleen length reduction and PGIC-assessed symptom improvement achieved at week 12 with ruxolitinib were sustained at week 24. Benefits in palpable spleen length reduction were sustained with longer-term ruxolitinib therapy (median follow-up: 149 weeks). At week 12, ruxolitinib treatment was associated with meaningful reductions in spleen volume by MRI or CT and WBC count in patients who achieved SPL and PGIC criteria, while worsening spleen volume and WBC count was observed with placebo (Figure 2); these effects were durable with longer-term therapy. Patients who achieved neither SPL nor PGIC criteria by week 12 (n = 47; 31.1%) achieved a clinically meaningful reduction in spleen volume by MRI or CT (mean change, −25.6%) and decreased WBC counts (mean change, −7.4%) at week 12 (Figure 2). Patients who did not achieve any palpable spleen reduction or PGIC-assessed symptom improvement (n = 13; 8.6%) were generally older, more likely to have high-risk MF as assessed by the International Prognostic Scoring System, and had higher WBC counts, lower platelet counts, and worse fibrosis grade at baseline compared with those achieving defined palpable spleen length reduction and/or PGIC-assessed symptom improvement with ruxolitinib.
Conclusions: Nearly all patients treated with ruxolitinib achieved some clinical benefit as assessed by practical measures of assessing efficacy (ie, palpable spleen reduction or PGIC-assessed symptom improvement) within 12 weeks of treatment initiation. Meaningful reductions in spleen volume and WBC count were observed in patients receiving ruxolitinib whether or not they achieved palpable spleen reduction by IWG-MRT/ELN consensus criteria or PGIC symptom improvement (“much improved” or “very much improved”) at week 12; these reductions were durable with long-term ruxolitinib therapy.
Miller:Incyte Corporation: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Komrokji:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding. Mesa:CTI: Research Funding; NS Pharma: Research Funding; Gilead: Research Funding; Incyte Corporation: Research Funding; Celgene: Research Funding. Sun:Incyte Corporation: Employment. Montgomery:Incyte Corporation: Employment. Verstovsek:Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal