Abstract
Background: Polycythemia vera (PV) is characterized by erythrocytosis and overactive JAK/STAT activity. The RESPONSE trial compared ruxolitinib (RUX), a JAK1/JAK2 inhibitor, with best available therapy (BAT) in patients (pts) with PV intolerant of or resistant to hydroxyurea according to modified European LeukemiaNet criteria. RUX was superior to BAT in achieving the primary endpoint (21% vs 1%; P<0.0001), and 60% of pts randomized to RUX achieved protocol-defined hematocrit (HCT) control through Wk 32 vs 20% of pts randomized to BAT (J Clin Oncol32:5s, 2014; suppl, abstract 7026). However, the actual phlebotomy rate between Wks 8−32 was only 20% in pts randomized to RUX compared with 62% in pts randomized to BAT, and 85% of pts in the RUX arm continued to receive treatment at the median 81-wk follow-up, suggesting most pts derived some benefit from RUX. Therefore, an analysis was conducted to evaluate the clinical efficacy of RUX in pts who did and did not achieve protocol-defined HCT control.
Methods: Phlebotomy-dependent PV pts with splenomegaly, aged ≥18 years, and resistant to or intolerant of HU were enrolled. Pts were required to have HCT between 40%−45% 14 days prior to randomization; those who did not could enter a phlebotomy control period to achieve a HCT in this range within 14 days of randomization. Eligible pts were randomized 1:1 to RUX or BAT. BAT pts could cross over to receive RUX from Wk 32 if they had not met the primary endpoint, or after Wk 32 due to protocol-defined disease progression. The primary composite endpoint comprised HCT control and a ≥35% reduction from baseline in spleen volume (SV) at Wk 32. HCT control was defined as lack of phlebotomy eligibility between Wks 8−32 with no more than 1 phlebotomy eligibility between randomization and Wk 8. Phlebotomy eligibility was based on protocol-defined HCT values (regardless of receipt of phlebotomy), and pts with missing data or assessments outside of protocol-defined time windows were considered non-responders. In pts who were HCT control non-responders (HCT-N) as defined by protocol, time to second phlebotomy eligibility was evaluated. For this analysis, pts without phlebotomy eligibility were excluded and phlebotomies in the first 8 wks were not considered. Patient-reported outcomes were evaluated in both HCT control responders (HCT-R) and HCT-N, including the 14-item modified Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) and the Patient Global Impression of Change (PGIC).
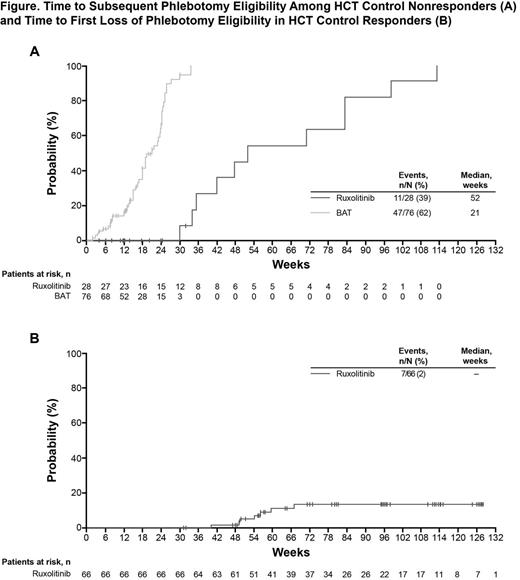
Results: Of 222 randomized pts (RUX, n=110; BAT, n=112), most were male (RUX, 60%; BAT, 71%) and the median age (range) was similar between treatment arms (RUX, 62.0 [34.0–90.0]; BAT, 60.0 [33.0–84.0]). Although pts had a HCT between 40% and 45% within 14 days prior to randomization (with or without phlebotomy), 24% of pts had a HCT >45% on Day 1 (similar between treatment arms) illustrating that many pts have poor HCT control over short intervals of observation. RUX treatment resulted in long-term benefits on HCT levels, even in pts who did not achieve protocol-defined HCT control through Wk 32. Among the RUX HCT-R group, the probability of maintaining HCT response was 97% at 48 wks and 87% at 80 wks. Most BAT pts crossed over to receive RUX immediately after the Wk 32 visit (84% between Wks 32 and 48). In the RUX HCT-N pts, the median time to subsequent phlebotomy eligibility was 52 wks, compared with 21 wks for BAT HCT-N pts (Figure). Compared with the entire BAT group, more RUX-R and RUX-N pts had a ≥50% improvement in the MPN-SAF total symptom score at Wk 32 (RUX-N, 38%; RUX-R, 40%; BAT, 4%). Median changes from baseline at Wk 32 in key symptoms such as tiredness (RUX-N; −50%; RUX-R, −49%; BAT, −4%), itching (RUX-N, −88%; RUX-R, −97%; BAT, −2%), and night sweats (RUX-N, −100%; RUX-R, −97%; BAT, 4%) were also greater among RUX-R and RUX-N pts than BAT pts. RUX-N and RUX-R pts were also more likely to consider their symptoms to be “much improved” or “very much improved” on the PGIC compared with BAT pts (RUX-N, 57%; RUX-R, 74%; BAT, 13%).
Conclusion: Among pts receiving RUX who did not achieve protocol-defined HCT control, the median time to subsequent phlebotomy eligibility was 1 year. In contrast, pts on BAT had a median time to subsequent phlebotomy eligibility of 21 wks. Furthermore, pts in the RUX arm achieved meaningful improvements in PV-related symptoms, regardless of meeting the endpoint of HCT control, while pts treated with BAT showed worsening or no improvement.
Verstovsek:Incyte Corporation: Research Funding. Off Label Use: Ruxolitinib is a JAK1/JAK2 inhibitor approved for the treatment of patients with intermediate or high-risk myelofibrosis, including primary myelofibrosis, post polycythemia vera myelofibrosis, and post-essential thrombocythemia myelofibrosis. Kiladjian:Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Mesa:Incyte Corporation: Research Funding; CTI: Research Funding; Gilead: Research Funding; Genentech: Research Funding; Eli Lilly: Research Funding; Promedior: Research Funding; NS Pharma: Research Funding; Sanofi: Research Funding; Celgene: Research Funding. Jones:Incyte Corporation: Employment, Equity Ownership. He:Incyte Corporation: Employment, Equity Ownership. Li:Novartis Pharmaceuticals: Employment, Equity Ownership. Habr:Novartis Pharmaceuticals: Employment, Equity Ownership. Vannucchi:Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal