Abstract
Introduction: Processing of peripheral blood progenitor cells (PBPC) for clinical transplantation or research applications aims to effectively isolate or deplete specific cell populations. We have previously reported the use of a novel ultrasound-based sorting technology, called acoustophoresis, for sorting of platelets (Dykes et al., PloS one 2011) and CD4+ cells from PBPC products (Lenshof et al., Cytometry Part A 2014). Here, we investigated the performance of microfluidic acoustophoresis for the separation of CD8+ lymphocytes from PBPC, and present a method for affinity-bead-mediated acoustic separation of cells which otherwise cannot be discriminated acoustically. In an acoustic standing wave field radiation forces induce movement of particles depending on particle and medium properties, such as for example particle size, density and compressibility. Targeting of cells by affinity specific beads generates cell-bead complexes that exhibit distinct acoustic properties relative to non-targeted cells and, thus, become possible to isolate.
Method: PBPC samples (n=16) were obtained from patients and healthy donors. Following density gradient centrifugation, mononuclear cells were labelled with anti-CD8 microbeads (Dynal) and sorted either on an acoustophoresis-microchip (Figure 1) or standard magnetic cell sorting technique for comparison. PBPC samples, target and waste fractions were analysed for purity, separation efficiency, recovery, T-cell function and progenitor cell content.
Results: PBPC products contained a mean of 11.6 ± 7.1% CD8+ cells before sorting. Purities obtained with acoustic sorting of CD8+ lymphocytes were 93.3 ± 6.8% compared to 94.4 ± 8.6% for magnetic sorting (n=16). Viabilities of sorted cells were 97.0 ± 3.9% (acoustic) and 97.5 ± 3.5% (magnetic). Mean separation efficiency recovery of acoustic sorted CD8+ cells was 57 ± 19% of the total CD8+ cells compared to a median recovery of magnetic sorted CD8+ cells of 43 ± 17%. Leukocyte subpopulation analysis performed after CD8 selection showed a relative increase of CD4 cells in the non-target fractions due to the removal of CD8 cells. Functional testing of sorted CD8+ lymphocytes showed unimpaired mitogen mediated proliferation capacity after 2-day, 4-day and 6-day stimulation with CD3/CD28. Furthermore, hematopoietic progenitor cell assays revealed a preserved colony forming ability of the post-sorted non-target cells
Conclusion: Acoustophoresis is a promising technology to efficiently sort bead-labelled lymphocyte populations from PBPC samples with high purity and recovery without impairing lymphocyte function. Affinity-bead acoustophoresis is, thus, an interesting technology for stem cell processing in PBPC.
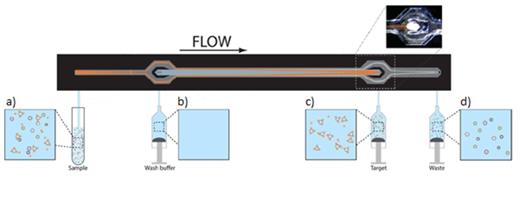
Picture of the acoustophoresis platform. The cell suspension with bead-labeled CD8+ cells enters through the side inlets (a) while the wash buffer (Histopaque-1077) is injected through the center inlet (b). Radiation forces in the acoustic standing wave field move the cell-bead complex faster to the center compared to non-target cells and can be separated in the center outlet of the channel (c). Non-target cells exit through the side outlets (d). The total length of the acoustophoresis microchip is 35mm.
Picture of the acoustophoresis platform. The cell suspension with bead-labeled CD8+ cells enters through the side inlets (a) while the wash buffer (Histopaque-1077) is injected through the center inlet (b). Radiation forces in the acoustic standing wave field move the cell-bead complex faster to the center compared to non-target cells and can be separated in the center outlet of the channel (c). Non-target cells exit through the side outlets (d). The total length of the acoustophoresis microchip is 35mm.
Laurell:Acousort AB: shareholder Other. Scheding:Acousort AB: Co-founder and board member Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal