Abstract
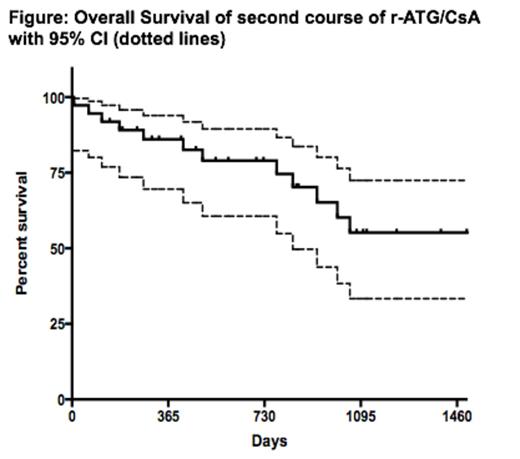
In patients with severe acquired aplastic anemia (AA) not eligible for hematopoietic stem cell transplant (HSCT), immunosuppression with horse antithymocyte globulin (h-ATG) and cyclosporine (CsA) is standard and is associated with a high response rate of about 60-70% at 6 months (Scheinberg et al. N Engl J Med 365: 430, 2011). Patients who are unresponsive to initial h-ATG can be rescued with rabbit ATG (r-ATG) in a third of cases (Scheinberg et al. Br J Haematol 133: 622, 2006). Since 2007, h-ATG is no longer available in most Latin American, Asian and European countries, with rabbit ATG (r-ATG) the only ATG formulation available in these markets. However, the outcome with r-ATG as first therapy in SAA is significantly inferior to that of h-ATG with worst response rate and overall survival (Scheinberg et al. N Engl J Med 365: 430, 2011). The salvage rate for patients who failed initial r-ATG is low with alemtuzumab and horse ATG [Scheinberg et al. Blood 119: 345, 2012; Am J Hematol 89: 467, 2014]. However, the salvage rate of a repeat course of r-ATG after initial r-ATG is unknown. Thus, we conducted a retrospective analysis in marrow failure referral Brazilian and Argentinian centers to address this question. The primary endpoint was hematologic response at 3 and 6 months, which was defined as transfusional independence and no longer meeting criteria for severe AA. Secondary endpoints included relapse, clonal evolution, and overall survival. Since 2005, 37 patients (32 refractory and 5 relapsed; 57% males, median age, 17 years; range 3-63 years) were re-treated with r-ATG (Thymoglobuline¨, Genzyme, Cambridge, MA, USA) with median dose of 3.5 mg/kg/d (range 1.67-5.0) for 5 days and oral cyclosporine adjusted to maintain blood levels between 150 and 400 ng/mL for 6 months. Corticosteroids, usually methylprednisolone, were given for at least 2 weeks to prevent serum sickness and trimethoprim–sulfamethoxazole was administered as Pneumocystis jiroveci prophylaxis. Only patients that completed the 5 day r-ATG course were included in the analysis. Second treatment was administered at a median of 283 days from first r-ATG/CsA (range 118-2379 days). After a median follow-up of 726 days (range 7-2320 days), the overall response rates at 3 and 6 months for initial r-ATG refractory patients was 5/32 (16%) and 7/32 (22%), respectively, and for those who relapsed 60% (3/5) (Table). Among all responders, 2 (20%) relapsed at 170 and 897 days after second treatment. In total, clonal evolutions were observed in 6 patients; 5 in non-responders (4 to monosomy 7; 1 trisomy 8 and 21), and 1 in a responder (myelodysplastic syndrome with normal karyotype). Three non-responders underwent matched-unrelated donor allogeneic HSCT. Twelve patients died, all who were non-responders to repeat r-ATG. Overall survival at 4.1 years (censored at the time of HSCT) was 58% (95% CI, 36-75%) (Figure). Our findings indicate that only a minority of r-ATG refractory AA patients may be successfully rescued with a second course of the same immunosuppression. Similar low salvage rates have been reported with alemtuzumab and h-ATG for those refractory to initial r-ATG. In the aggregate, these data show that: 1) other therapies should be considered for those refractory to initial r-ATG such as alternative donor HSCT, thrombopoietin agonists, or other experimental therapies; 2) the high success rate of initial h-ATG therapies cannot be recapitulated when r-ATG is administered first given the low salvage rate with alemtuzumab, h-ATG and now (current data) with r-ATG; 3) for patients that relapse after first r-ATG treatment, second course r-ATG may be a reasonable option.
Hematologic response at 3 and 6 months to second course of rabbit ATG plus cyclosporine
| . | Refractory to first r-ATG/CsA (n=32) . | 95% CI . | Relapsed to first r-ATG/CsA (n=5) . | 95% CI . |
|---|---|---|---|---|
| At 3 months no. (%) | 5 (16) | 3-28 | 3 (60) | 17-100 |
| At 6 months no. (%) | 7 (22) | 8-36 | 3 (60) | 17-100 |
| . | Refractory to first r-ATG/CsA (n=32) . | 95% CI . | Relapsed to first r-ATG/CsA (n=5) . | 95% CI . |
|---|---|---|---|---|
| At 3 months no. (%) | 5 (16) | 3-28 | 3 (60) | 17-100 |
| At 6 months no. (%) | 7 (22) | 8-36 | 3 (60) | 17-100 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal