Abstract
Introduction: Prophylaxis with replacement factor IX (FIX) therapies can reduce the frequency of bleeding episodes in patients with severe hemophilia B. There is currently a lack of data on factor activity levels in patients that treat bleeding episodes while adhering to a prophylactic regimen. The phase 3 B-LONG study (Powell et al. NEJM 2013) demonstrated that recombinant factor IX Fc fusion protein (rFIXFc) had a prolonged half-life relative to recombinant FIX, and that rFIXFc was efficacious and safe for prophylaxis and treatment of bleeding in subjects with hemophilia B; previous reports have evaluated treatment of bleeding in all subjects in B-LONG, including those receiving episodic treatment with rFIXFc. The purpose of this analysis was to assess the efficacy of rFIXFc for the treatment of bleeding in patients that received rFIXFc prophylaxis in the B-LONG study. Additionally, FIX activity levels were predicted under scenarios where treatment of bleeding occurred in close proximity to prophylactic doses using a population pharmacokinetic (PK) modeling approach.
Methods: B-LONG included previously treated male subjects ≥12 years of age that had moderately-severe to severe hemophilia B (≤2 IU/dL endogenous FIX activity). Treatment of bleeding was assessed in subjects receiving weekly prophylaxis (Arm 1) and individualized interval prophylaxis (Arm 2). The number of bleeds, median dose per infusion to treat a bleed, and number of infusions to resolve bleeds were evaluated. Predicted activity levels were generated for 1000 hypothetical patients using a three-compartment population PK model that was developed using PK data from B-LONG (n=123) and a phase 1/2a study of rFIXFc (n=12). Factor activity was predicted assuming that bleeds were treated with 50 IU/kg rFIXFc either 24 hours before or 24 hours after a scheduled prophylactic dose for patients on regimens of 50 IU/kg weekly (at steady-state). The treatment of bleeding dose was selected because it was in the recommended range in the B-LONG study and was consistent with the median dose per infusion used in B-LONG. The 50 IU/kg weekly regimen was the most common starting prophylactic regimen in B-LONG; 24 hours was selected to approximate the shortest time interval between separate treatment of bleeding and prophylactic doses.
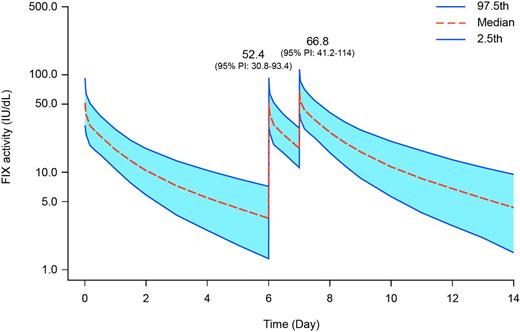
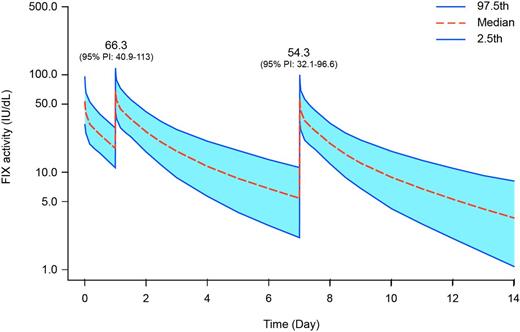
Results: In Arm 1 of B-LONG, bleeding episodes were reported in 47 of 61 subjects, and there were 167 episodes in total. In Arm 2, 67 episodes were reported in 15 of 26 subjects. The median annualized bleeding rate (ABR) was 2.95 in Arm 1 (median spontaneous ABR 1.04) and 1.38 in Arm 2 (median spontaneous ABR 0.88). In Arm 1, 85% of bleeding episodes resolved with 1 infusion and the median dose per infusion was 47.11 IU/kg (interquartile range [IQR] 31.75-56.03). In Arm 2, 85.1% of bleeding episodes resolved with 1 infusion and the median dose per infusion was 44.78 IU/kg (IQR 33.63-74.33). rFIXFc was generally well-tolerated, and there were no vascular thrombotic events reported in any arm of the B-LONG study. The predicted median Cmax from the population PK model was in the normal range for both scenarios (Table 1; Figure 1).
FIX activity over time for hypothetical patients that treat a bleeding episode while on prophylactic regimens. Graphs correspond to scenarios as labelled in Table 1.
FIX activity over time for hypothetical patients that treat a bleeding episode while on prophylactic regimens. Graphs correspond to scenarios as labelled in Table 1.
Conclusions: Predictions of factor activity from a population PK model showed that the vast majority of patients maintained factor activity within the normal range when rFIXFc was dosed in close proximity for both prophylaxis and to treat a bleeding episode. These predictions also indicated that maximum plasma concentration was achieved rapidly. An analysis of bleeding episodes in subjects specifically from the prophylaxis arms of B-LONG showed that rFIXFc was a safe and efficacious treatment for bleeding in patients on prophylactic regimens.
Powell:Bayer: Research Funding; Baxter Healthcare: Research Funding; Biogen Idec: Research Funding; CSL Behring: Research Funding; Novo Nordisk: Research Funding. Ozelo:Bayer: Research Funding; Baxter: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Research Funding; Novo Nordisk Haemophilia Foundation: Research Funding. Ragni:Pfizer: Research Funding; Novo Nordisk: Research Funding; Novartis: Research Funding; Merck: Research Funding; CSL Behring: Research Funding; Bayer: Research Funding; Baxter: Research Funding; Tacere Benitec: Consultancy, Drug Safety Monitoring Board, Drug Safety Monitoring Board Other; GlaxoSmithKline: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Biogen Idec: Consultancy, Research Funding; Spark Therapeutics: Research Funding; Vascular Medicine Institute, PIttsburgh, PA: Research Funding. Pasi:Bayer: Membership on an entity's Board of Directors or advisory committees; BPL: Membership on an entity's Board of Directors or advisory committees; Biogen Idec: Educational Support and Travel Grants, Educational Support and Travel Grants Other, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Educational Support and Travel Grants, Educational Support and Travel Grants Other; OctaPharma: Educational Support and Travel Grants Other, Membership on an entity's Board of Directors or advisory committees; Pfizer: Educational Support and Travel Grants Other, Membership on an entity's Board of Directors or advisory committees. Perry:Biogen Idec: Consultancy, Research Funding; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; Baxter: Honoraria. Zhu:Biogen Idec: Employment, Equity Ownership. Mei:Biogen Idec: Employment, Equity Ownership. Pierce:Biogen Idec: Employment, Equity Ownership. Li:Biogen Idec: Employment, Equity Ownership. Robinson:Biogen Idec: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal