Abstract
Background: Patients with hemophilia A (HA) and inhibitory antibodies are typically treated with bypassing agents if the inhibitor titer is >5 Bethesda units (BU). However, some patients with elevated inhibitor titers are treated with FVIII therapy in conjunction with immune tolerance regimens or in combination with bypassing agents. The Bethesda assay detects the inhibitory capacity for FVIII neutralization after 2 hours' incubation of patient's plasma mixed with pooled normal plasma (PNP). Nonetheless the inhibitory titer does not express the kinetic behavior of the inhibitor throughout the 2 hours of incubation. Furthermore, standard plasma derived FVIII is used for the Bethesda assay, whereas many patients are currently treated with recombinant FVIII products. Patients with severe HA who develop inhibitors typically have a polyclonal response. In vitro responses to monoclonal specific anti- epitope antibodies showed that group A anti-A2 antibodies ( high titer, type I antibodies) rapidly and completely inhibit FVIII and are not affected by the presence of Von Willebrand factor (VWF). In contrast, many anti- C1 and C2 antibodies compete with VWF for binding to FVIII. As patients' response to various FVIII sources (eg: with/ without VWF) may be affected by their unique B cell epitope profile, personalized individually tailored FVIII brand specific therapy may be considered.
Aims : We aimed to define our inhibitor patients' individual response to various FVIII sources by assessing the kinetics of FVIII inhibition and by patients' FVIII epitope specificity.
Methods: Plasma was obtained from HA patients with inhibitors. FVIII neutralization profile was determined against PNP, recombinant full length FVIII (rFVIII= Kogenate- FS, Bayer), plasma derived Von Willebrand containing FVIII (pdFVIII = Optivate, BPL) and correlated to the inhibitory (BU) titer. To test FVIII neutralization profile, patients' platelet poor plasma was either incubated with PNP or spiked with 2U/ml rFVIII/pdFVIII and sequential measurements of residual FVIII activity was recorded over 120 minutes. FVIII activity was measured by one stage aPTT assay. All inhibitor assays were performed on the same sample simultaneously. Results were compared to the standard Bethesda assay.
Domain-specific anti-FVIII antibody mapping was carried out by direct ELISA using purified single human domain hybrid FVIII protein. Patient plasma was serially diluted down the ELISA plate. Domain predominance was determined by comparison of resultant ELISA curves. In addition the presence of anti-A2 group A antibodies in the polyclonal patient plasma was assessed by competition ELISA.
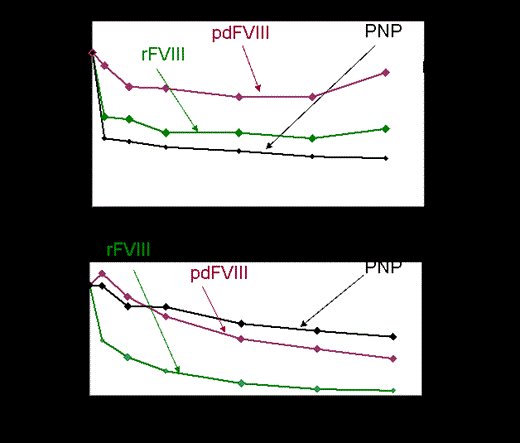
Results: Our patient cohort was heterogeneous, consisting of patients aged 3-70 years with inhibitor titers of 0.5-131 BU. No obvious correlation was found between kinetic profiles and the inhibitory BU titer. For each patient, variability of inhibitor activity against different FVIII sources was noted. In individual patients, discrepant inhibitory kinetics were identified when commercial FVIII (rFVIII or pd-FVIII) was added, compared to PNP, utilized for standard determination of inhibitory titer in BU/ml. Among patients with similar BU titer, different FVIII neutralization profile was noted. Furthermore, the source of FVIII yielded individual neutralization curves that did not correlate with the common gold standard of inhibitory (BU) titer (Figure). Correlation between patients' epitopes and their neutralization profile was difficult to assess as in most patients no single predominant inhibitory epitope could be defined. However, in patients with C2 predominance antibodies improved neutralization was detected with pd -FVIII as compared to rFVIII source. High titer patient plasmas that contained anti-A2 group A antibodies inhibited FVIII completely at the first time point regardless of the VWF content of the FVIII source.
Conclusion: The Bethesda assay neither predicts inhibitory kinetics nor defines patients' response to various FVIII sources. Our combination of assays may identify the best potential FVIII source for treatment of patients with inhibitory antibodies. We suggest that FVIII source dependent neutralization kinetics and epitope mapping may be applied as additional tools for future individual therapy tailoring. Larger sample sizes and repeated samples over time will be required to validate these results.
Dunn:CSL Behring,: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Baxter: Membership on an entity's Board of Directors or advisory committees; Biogen: Membership on an entity's Board of Directors or advisory committees; Pfiser: Membership on an entity's Board of Directors or advisory committees. Meeks:Grifols: Membership on an entity's Board of Directors or advisory committees; Bayer HealthCare: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Consultancy; Baxter: Membership on an entity's Board of Directors or advisory committees. Kenet:BPL: Research Funding; Opko Biologics (Prolor Biotech): Consultancy, Research Funding; Novonordisk: Honoraria; Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi Aventis: Membership on an entity's Board of Directors or advisory committees; Baxter: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal