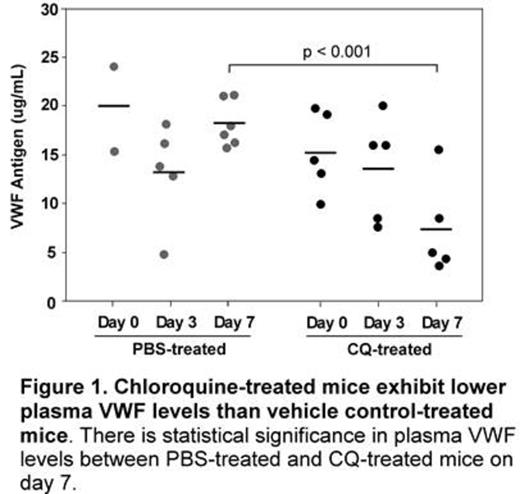
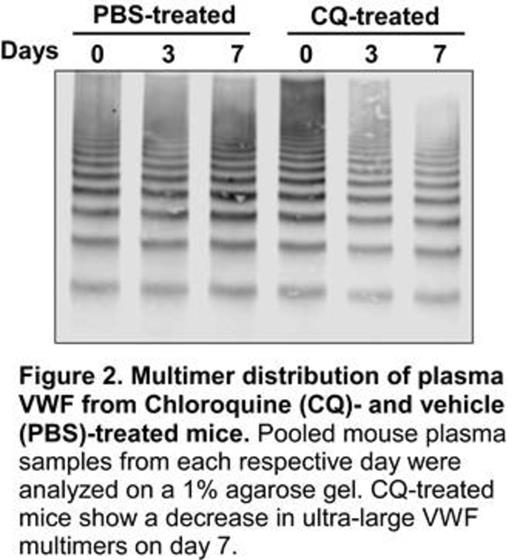
The regulation of von Willebrand factor (VWF) processing, packaging, and secretion is important for primary hemostasis. Recently, autophagy has been implicated in modulating VWF maturation and Weibel-Palade body (WPB) morphology. Additionally, treatment of mice and humans with chloroquine (CQ), an anti-malarial agent and pharmacological inhibitor of autophagy, is shown to increase bleeding time. Therefore, we hypothesize that targeting autophagic flux may be therapeutic for arterial thrombotic disorders such as thrombotic thrombocytopenic purpura (TTP). To test this hypothesis, we first treated human umbilical vein endothelial cells (HUVECs) with CQ in culture and showed that CQ dose-dependently decreased VWF antigen levels and multimer sizes in the conditioned medium of histamine-stimulated cells (not shown). Knockdown of an autophagy-related protein (Atg7) with shRNA in HUVECs had similar effect to CQ treatment in VWF secretion and multimer distribution (not shown). More interestingly, daily injections (i.p.) of CQ at 60 mg/kg into Adamts13-/- mice (CAST/Ei strain) for 7 days reduced plasma VWF concentration by more than 60% compared to vehicle control (Fig. 1). Interestingly, VWF secreted from CQ-treated mice remained functional as collagen binding activity/antigen ratio was comparable to vehicle control mice. However, multimer analysis demonstrated the selective lack of ultra large VWF in plasma of Adamts13-/- mice treated with CQ as compared with those treated with vehicle alone (Fig. 2). These results indicate that targeting autophagy pathway with a pharmacological agent, such as CQ, may modulate VWF secretion and, thereby, arterial thrombosis. Our ongoing experiments are to determine the therapeutic efficacy of CQ and other autophagy inhibitors in murine models of arterial thrombosis and TTP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal