Abstract
Background
Autosomal dominant germline mutations in the phosphatidylinositol-3-OH kinase (PIK3CD) encoding for the PI(3)K catalytic subunit p110δ, lead to combined immunodeficiency with increased incidence of B-cell lymphomas. (Lucas CL et.al. Nature Immunology 2014). While p110δ is selectively expressed in leukocytes, it is critical for TCR and BCR signaling and lymphocyte homeostasis. Clinically, these patients may present with sinopulmonary infections, bronchiectasis, cytomegalovirus (CMV) and/or Epstein-Barr virus (EBV) viremia, lymphoproliferation and autoimmune cytopenias. Immune phenotype includes naïve CD4+ T cell lymphopenia, expanded terminally differentiated or exhausted T cells, increased circulating transitional B cells and reduced class-switched memory B cells. Herein we report immunophenotypic abnormalities in B-lymphoid maturation in the bone marrow (BM) of 5 patients with PIK3CD mutations.
Methods
BM from 5 patients with PIK3CD mutations (2 males, 3 females, age range: 4–15 years, median 11.5 years) were studied by flow cytometry (FC), morphology and immunohistochemistry (IHC). BM aspirate from 5 healthy age matched pediatric patients were used as controls for flow cytometric analysis of B-cell subsets and maturation. Antibodies against CD45, CD3, CD4, CD8, CD19, CD10, CD34, CD20, and surface kappa and lambda light chains were used for FC. B-lymphocyte subsets were defined as: early stage precursor B-cells (CD34+/CD19+/CD10+bright/CD20-); intermediate precursor B-cells (CD45+moderate/ CD19+/CD10+moderate/CD34-); and late stage and mature B cells (CD34-/CD10-/CD19+/CD45+bright/CD20+). The intermediate subset corresponds to transitional B cells (developmentally intermediate between immature and mature naive B cells). IHC and in situ hybridization staining were applied to biopsy sections using standard methods. Prism software was used for statistical analyses (Mann-Whitney test).
Results
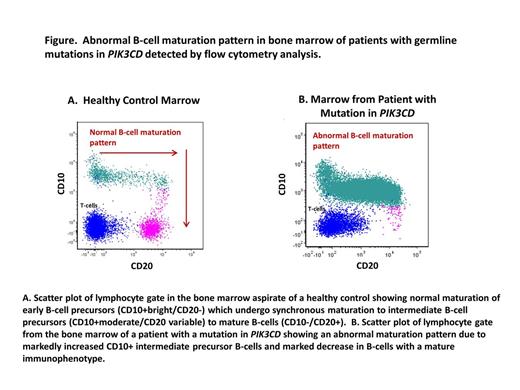
There was no significant difference in the median percentage of early B-cell precursors (among all B-lymphocytes) between the PIK3CD patients and the age-matched controls (3.6% vs. 3.7%; p=0.8). However, all PIK3CD marrows showed expanded CD10+ intermediate precursor B-cells which were overall 2.5 times more abundant in PIK3CD marrows than in controls (94.6% vs. 37.4% of all B-cells; p<0.01). Additionally, the PIK3CD patients showed a marked reduction in mature B-cells with 29 times fewer mature CD20+/CD10- B-cells than controls (2% vs 57%; p<0.01). These differences resulted in a markedly abnormal B-cell maturation pattern in all PIK3CD patients (Figs. A and B). A subset of CD10+ and bright CD20+ B-cells expressed polytypic light chains in the PIK3CD marrows. The median CD4:CD8 T-cell ratio was 0.32 in PIK3CD marrows with markedly reduced CD4+ T-cells.
BM core biopsies showed overall normal cellularity with increased lymphocytes (20-30% of the cellular marrow). IHC revealed increased CD20+ lymphocytes (15-20% of all nucleated cells) and CD10+ lymphocytes showed similar distribution suggesting coexpression with CD20. TdT and CD34 highlighted approximately 5% of all nucleated cells. CD138, and kappa and lambda light chains showed unremarkable scattered polytypic plasma cells. CD3+ and CD8+ T-cells accounted for 5-10% of BM cells and CD4+ lymphocytes were reduced. EBV was positive in one case. CMV was negative in all cases.
Conclusions
For the first time, we report B-cell maturation abnormalities in the bone marrow of patients with germline mutations in PIK3CD. All marrows showed an abnormal pattern of B cell maturation characterized by an absolute increase in CD10+ intermediate precursor B-cells and a marked decrease in mature B-cells. The findings suggest either a partial block in B-cell late stage maturation or other mechanism leading to increased CD10+ B-cell precursors and markedly reduced mature B-cells. Lymphoid hyperplasia and lymphoma have been described in PIK3CD patients. The increased CD10+ B cell precursors and the abnormal maturation pattern noted by flow cytometry may mimic CD10+ B-cell neoplasia (e.g. acute lymphoblastic leukemia or Burkitt lymphoma) but detailed analysis showed no morphologic or immunophenotypic evidence of B-cell neoplastic involvement in any of the five patients studied.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal