Abstract
Introduction: The pan-selectin antagonist rivipansel (GMI-1070) reduced intravascular arrest of red/white blood cell aggregates and improved blood flow and survival in a mouse model of sickle cell disease vaso-occlusive crisis (SCD VOC). In a Phase 1 study of GMI-1070 infusion in SCD adults at steady state (not in VOC), GMI-1070 decreased markers of cellular activation including neutrophil integrins, platelet/neutrophil aggregates, soluble adhesion molecule concentration; and markers of hemostatic activation. Furthermore, in a randomized Phase 2 study of SCD patients, treatment of VOC with GMI-1070 improved clinical outcomes such as time to resolution of crisis, time to discharge, and IV opioid use. Herein we report on the effect of GMI-1070 on biomarkers of cellular and hemostatic cascade activation from this Phase 2 trial.
Methods: Patients in VOC enrolled in a prospective, randomized multi-center double-blind Phase 2 trial, ages 12-60 with HbSS or HbSB0thalassemia were treated with GMI-1070 q12h or placebo, in addition to standard treatment per institutional practice, until resolution of VOC. Clinical outcomes and pharmacokinetics have been previously reported (ASH 2013 Abstracts 775, 776, and 2206). Biomarker blood samples were drawn prior to study drug, and on a sparse sampling basis at times starting 30 minutes after initial dose and continuing until 36 hours after the last dose. Analytes measured included: soluble adhesion molecules E-selectin (sEsel), P-selectin, L-selectin, intercellular adhesion molecules 1 and 3, vascular cell adhesion molecule-1; and tissue factor and thrombin-antithrombin complexes by ELISA. At some sites, surface expression of monocyte b2 integrins MAC-1 & LFA-1 and platelet-monocyte aggregates were also measured by flow cytometry. Comparisons were made between the GMI-1070 and placebo groups, and serial expression levels were compared over time. Subgroup analyses were performed by hydroxyurea (HU) use, age group, baseline WBC, and responders' based on clinical outcomes. A mixed effect model was used to test the LS means difference at each time point and ANCOVA model was used to analyze the nadir, peak, and last dose values.
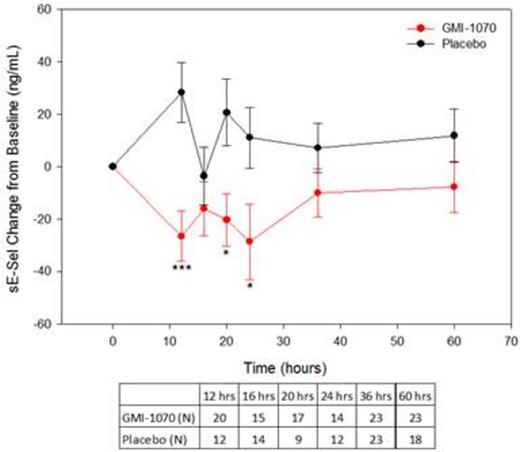
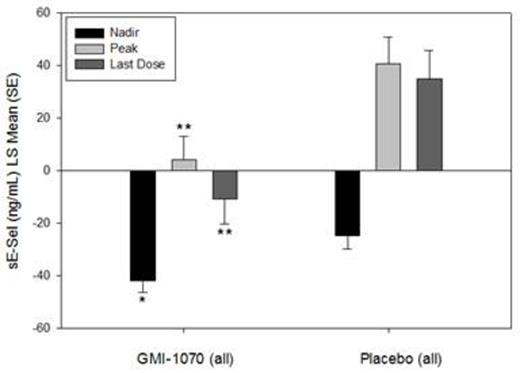
Results: ELISA and flow cytometry samples were collected from 70 and 15 subjects, respectively. Soluble E-selectin levels were reduced for the group on GMI-1070 compared to placebo throughout hospitalization, and the differences were statistically significant at some time-points (Figure 1). Baseline sEsel levels were similar; but the peak, nadir, and level at last dose were all lower in the GMI-1070 group (Figure 2). Exploratory subgroup analysis by HU use, age group, response as measured by visual analog scale or opiate use, frequency of VOC in the past, and baseline white blood cell count revealed consistently lower sEsel levels in the GMI-1070 group. Many, but not all, of these differences reached statistical significance.
Conclusion: GMI-1070 use during VOC resulted in consistent and significant reductions of sEsel, overall and in sub-groups as compared to placebo. These findings are consistent with the hypothesized effect of GMI-1070 on endothelial activation and/or apoptosis, mediated by inhibition of E-selectin, although an effect on sEsel clearance cannot be excluded. A Phase 3 study is planned to evaluate efficacy and safety of GMI-1070 as treatment for VOC. Soluble E-selectin concentrations may be useful as a biomarker of pharmacodynamic effect.
sE-sel was reduced in the GMI-1070 group at all timepoints tested. Comparison to placebo for change from baseline over time is shown. *p<0.05 ***p<0.001
sE-sel was reduced in the GMI-1070 group at all timepoints tested. Comparison to placebo for change from baseline over time is shown. *p<0.05 ***p<0.001
Figure 2: sE-sel was similar at baseline between GMI-1070 and placebo groups; mean change was different for GMI-1070 compared to placebo at the nadir, peak, and last dose values. Change from baseline is shown. *p<0.05 **p<0.01
Figure 2: sE-sel was similar at baseline between GMI-1070 and placebo groups; mean change was different for GMI-1070 compared to placebo at the nadir, peak, and last dose values. Change from baseline is shown. *p<0.05 **p<0.01
Wun:GlycoMimetics: Research Funding; Pfizer: Steering Committee, Steering Committee Other; Emmaus Medical: Consultancy. Telen:GlycoMimetics: Research Funding; Pfizer: Consultancy; Dilaforette: Research Funding. Krishnamurti:GlycoMimetics: Research Funding. McCavit:GlycoMimetics: Research Funding; Pfizer: Consultancy. DeCastro:GlycoMimetics: Research Funding. Flanner:GlycoMimetics: Employment, Equity Ownership. Kuypers:GlycoMimetics: Research Funding. Larkin:GlycoMimetics: Research Funding. Rhee:GlycoMimetics: Consultancy. Magnani:GlycoMimetics Inc.: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Thackray:GlycoMimetics: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal